Periodontitis, chronic liver diseases, and the emerging oral-gut-liver axis
- PMID: 35244954
- PMCID: PMC9314012
- DOI: 10.1111/prd.12427
Periodontitis, chronic liver diseases, and the emerging oral-gut-liver axis
Abstract
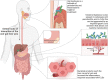
The liver carries out a wide range of functions ranging from the control of metabolites, nutrient storage, and detoxification to immunosurveillance. While inflammation is essential for the tissue remodeling and maintenance of homeostasis and normal liver physiology, constant exposure to dietary and microbial products creates a niche for potentially prolonged immune activation and unresolved inflammation in susceptible host. Failure to restrain inflammation can lead to development of chronic liver diseases characterized by fibrosis, cirrhosis and eventually liver failure. The liver maintains close interactions with numerous organs which can influence its metabolism and physiology. It is also known that oral cavity microenvironment can influence the physiological conditions of other organs and emerging evidence implicates that this could be true for the liver as well. Presence of chronic inflammation and dysbiotic microbiota is a common feature leading to clinical pathology both in periodontitis and chronic liver diseases (CLDs). In fact, known CLDs appear to have some relationship with periodontitis, which impacts the onset or progression of these conditions in a bidirectional crosstalk. In this review, we explore the emerging association between oral-gut-liver axis focusing on periodontitis and common CLDs including nonalcoholic fatty liver disease, chronic viral hepatitis, liver cirrhosis, and hepatocellular cancer. We highlight the immune pathways and oral microbiome interactions which can link oral cavity and liver health and offer perspectives for future research.
Keywords: chronic viral hepatitis; cirrhosis; fatty liver disease; hepatocellular cancer; liver; periodontal; periodontitis.
© 2022 The Authors. Periodontology 2000 published by John Wiley & Sons Ltd.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

