Specific mesoderm subset derived from human pluripotent stem cells ameliorates microvascular pathology in type 2 diabetic mice
- PMID: 35245116
- PMCID: PMC8896785
- DOI: 10.1126/sciadv.abm5559
Specific mesoderm subset derived from human pluripotent stem cells ameliorates microvascular pathology in type 2 diabetic mice
Abstract
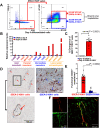
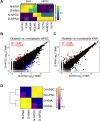
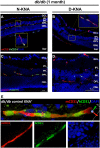
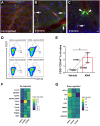
Human induced pluripotent stem cells (hiPSCs) were differentiated into a specific mesoderm subset characterized by KDR+CD56+APLNR+ (KNA+) expression. KNA+ cells had high clonal proliferative potential and specification into endothelial colony-forming cell (ECFCs) phenotype. KNA+ cells differentiated into perfused blood vessels when implanted subcutaneously into the flank of nonobese diabetic/severe combined immunodeficient mice and when injected into the vitreous of type 2 diabetic mice (db/db mice). Transcriptomic analysis showed that differentiation of hiPSCs derived from diabetics into KNA+ cells was sufficient to change baseline differences in gene expression caused by the diabetic status and reprogram diabetic cells to a pattern similar to KNA+ cells derived from nondiabetic hiPSCs. Proteomic array studies performed on retinas of db/db mice injected with either control or diabetic donor-derived KNA+ cells showed correction of aberrant signaling in db/db retinas toward normal healthy retina. These data provide "proof of principle" that KNA+ cells restore perfusion and correct vascular dysfunction in db/db mice.
Figures



References
-
- Carcamo-Orive I., Hoffman G. E., Cundiff P., Beckmann N. D., D’Souza S. L., Knowles J. W., Patel A., Papatsenko D., Abbasi F., Reaven G. M., Whalen S., Lee P., Shahbazi M., Henrion M. Y. R., Zhu K., Wang S., Roussos P., Schadt E. E., Pandey G., Chang R., Quertermous T., Lemischka I., Analysis of transcriptional variability in a large human iPSC library reveals genetic and non-genetic determinants of heterogeneity. Cell Stem Cell 20, 518–532.e9 (2017). - PMC - PubMed
-
- Park T. S., Zimmerlin L., Evans-Moses R., Thomas J., Huo J. S., Kanherkar R., He A., Ruzgar N., Grebe R., Bhutto I., Barbato M., Koldobskiy M. A., Lutty G., Zambidis E. T., Vascular progenitors generated from tankyrase inhibitor-regulated naïve diabetic human iPSC potentiate efficient revascularization of ischemic retina. Nat. Commun. 11, 1195 (2020). - PMC - PubMed
-
- Murry C. E., Keller G., Differentiation of embryonic stem cells to clinically relevant populations: Lessons from embryonic development. Cell 132, 661–680 (2008). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

