Neutralization and durability of 2 or 3 doses of the BNT162b2 vaccine against Omicron SARS-CoV-2
- PMID: 35245438
- PMCID: PMC8853806
- DOI: 10.1016/j.chom.2022.02.015
Neutralization and durability of 2 or 3 doses of the BNT162b2 vaccine against Omicron SARS-CoV-2
Abstract
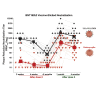
Two doses of the BNT162b2 mRNA vaccine are highly effective against SARS-CoV-2. Here, we tested the antibody neutralization against Omicron SARS-CoV-2 after 2 and 3 doses of BNT162b2. Serum from vaccinated individuals was serially tested for its ability to neutralize wild-type SARS-CoV-2 (USA-WA1/2020) and an engineered USA-WA1/2020 bearing the Omicron spike glycoprotein. At 2 or 4 weeks post dose 2, the neutralization geometric mean titers (GMTs) against the wild-type and Omicron-spike viruses were 511 and 20, respectively; at 1 month post dose 3, the neutralization GMTs increased to 1,342 and 336; and at 4 months post dose 3, the neutralization GMTs decreased to 820 and 171. The data support a 3-dose vaccination strategy and provide a glimpse into the durability of the neutralization response against Omicron.
Keywords: BNT162b2 vaccine; Pfizer vacine; SARS-CoV-2; neutralization; neutralization durability; variants.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests X.X. and P.-Y.S. have filed a patent on the reverse genetic system of SARS-CoV-2. H.X., J.Z., C.K., X.X., and P.-Y.S. received compensation from Pfizer to perform the project. H.C., Q.Y., M.C., D.C., K.U.J., and K.A.S. are employees of Pfizer and may hold stock options. A.M. is an employee of BioNTech and may hold stock options.
Figures

References
-
- Cele S., Jackson L., Khan K., Khoury D., Moyo-Gwete T., Tegally H., Scheepers C., Amoako D., Karim F., Bernstein M., et al. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv. 2021 doi: 10.1101/2021.12.08.21267417. Preprint at. - DOI
-
- Dormitzer P.R., Suphaphiphat P., Gibson D.G., Wentworth D.E., Stockwell T.B., Algire M.A., Alperovich N., Barro M., Brown D.M., Craig S., et al. Synthetic generation of influenza vaccine viruses for rapid response to pandemics. Sci. Transl. Med. 2013;5:185ra68. doi: 10.1126/scitranslmed.3006368. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

