Mechanisms and pathogenesis of chronic rhinosinusitis
- PMID: 35245537
- PMCID: PMC9081253
- DOI: 10.1016/j.jaci.2022.02.016
Mechanisms and pathogenesis of chronic rhinosinusitis
Abstract
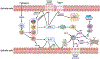
Chronic rhinosinusitis (CRS) is a heterogeneous disease characterized by local inflammation of the upper airways and is historically divided into 2 main phenotypes: CRS with nasal polyps and CRS without nasal polyps. Inflammation in CRS is mainly characterized by 3 endotypes based on elevation of canonical lymphocyte cytokines: type (T) 1 (T1) by TH1 cytokine IFN-γ, T2 by TH2 cutokines IL-4, IL-5, and IL-13, and T3 by TH17 cytokines including IL-17. Inflammation in both CRS without nasal polyps and CRS with nasal polyps is highly heterogeneous, and the frequency of various endotypes varies geographically around the world. This finding complicates establishment of a unified understanding of the mechanisms of pathogenesis in CRS. Sinonasal epithelium acts as a passive barrier, and epithelial barrier dysfunction is a common feature in CRS induced by endotype-specific cytokines directly and indirectly. The sinonasal epithelium also participates in both innate immunity via recognition by innate pattern-recognition receptors and promotes and regulates adaptive immunity via release of chemokines and innate cytokines including thymic stromal lymphopoietin. The purpose of this review was to discuss the contribution of the epithelium to CRS pathogenesis and to update the field regarding endotypic heterogeneity and various mechanisms for understanding pathogenesis in CRS.
Keywords: Chronic rhinosinusitis; endotype; eosinophils; epithelial dysfunction; nasal polyps; neutrophils.
Copyright © 2022 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures

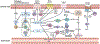
References
-
- Orlandi RR, Kingdom TT, Hwang PH, Smith TL, Alt JA, Baroody FM, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol 2016; 6 Suppl 1:S22–209. - PubMed
-
- Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020; 58:1–464. - PubMed
-
- Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 2006; 61:1280–9. - PubMed
-
- Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol 2008; 122:961–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

