The interplay of viral loads, clinical presentation, and serological responses in SARS-CoV-2 - Results from a prospective cohort of outpatient COVID-19 cases
- PMID: 35245784
- PMCID: PMC8855229
- DOI: 10.1016/j.virol.2022.02.002
The interplay of viral loads, clinical presentation, and serological responses in SARS-CoV-2 - Results from a prospective cohort of outpatient COVID-19 cases
Abstract
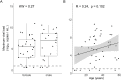
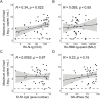
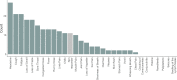
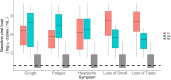
Risk factors for disease progression and severity of SARS-CoV-2 infections require an understanding of acute and long-term virological and immunological dynamics. Fifty-one RT-PCR positive COVID-19 outpatients were recruited between May and December 2020 in Munich, Germany, and followed up at multiple defined timepoints for up to one year. RT-PCR and viral culture were performed and seroresponses measured. Participants were classified applying the WHO clinical progression scale. Short symptom to test time (median 5.0 days; p = 0.0016) and high viral loads (VL; median maximum VL: 3∙108 copies/mL; p = 0.0015) were indicative for viral culture positivity. Participants with WHO grade 3 at baseline had significantly higher VLs compared to those with WHO 1 and 2 (p = 0.01). VLs dropped fast within 1 week of symptom onset. Maximum VLs were positively correlated with the magnitude of Ro-N-Ig seroresponse (p = 0.022). Our results describe the dynamics of VLs and antibodies to SARS-CoV-2 in mild to moderate cases that can support public health measures during the ongoing global pandemic.
Keywords: COVID-19; Immune response; Public health; RT-PCR; SARS-CoV-2; Serological testing; Viral culture.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
AW and MH (on the behalf of the institute) report personal fees and non-financial support from Roche Diagnostics, LO reports non-financial support from Roche Diagnostics. AW, MH and LO report non-financial support from Euroimmun, non-financial support from Viramed, non-financial support from Mikrogen. AW, MH, LO report grants, non-financial support and other from German Center for Infection Research (DZIF), grants and non-financial support from Government of Bavaria, non-financial support from BMW, non-financial support from Munich Police, non-financial support and other from Accenture. MH and AW report non-financial support from Dr. Becker MVZ during the conduct of the study. In addition, MH and AW have a patent on a sample system for sputum diagnostics of SARS-CoV-2 pending.
Figures




References
-
- He X., et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26(5):672–675. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

