A systematic review and meta-analysis of the accuracy of SARS-COV-2 IGM and IGG tests in individuals with COVID-19
- PMID: 35245882
- PMCID: PMC8863416
- DOI: 10.1016/j.jcv.2022.105121
A systematic review and meta-analysis of the accuracy of SARS-COV-2 IGM and IGG tests in individuals with COVID-19
Abstract
Introduction: Active SARS-CoV-2 infection is confirmed mainly through the detection of viral nucleic acid via the reverse transcriptase polymerase chain reaction (RT-PCR) technique. Methods to assess humoral responses contribute to the monitoring of the disease and confirmation of exposure to the virus.
Objective: To evaluate the accuracy of tests for IgM and IgG antibodies for SARS-CoV-2 infection confirmed by RT-PCR and utility as complementary data for immunosurveillance.
Methods: Literature research was performed by searching the terms "COVID-19", "COVID-19 diagnostic testing" and "test" in the databases MEDLINE, EMBASE, Cochrane Library, Web of Science and Cumulative Index to Nursing and Allied Health Literature to search for potentially eligible observational studies without language restrictions published up to September 2020.
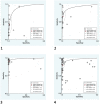
Results: The pooled sensitivity and specificity, regardless of collection moment, was 80.0% (CI 95% 72.0-86.0) and 97.0% (CI 95% 94.0-98.0) for "IgM and/or IgG", respectively. Serology considering immunoglobulins M and G together had a high accuracy performance on "fifteenth day and after": sensitivity and specificity was 91.0% (CI 95% 85.0-94.0) and 98.0% (CI 95% 95.0-99.0) respectively, DOR 461 and AUC 0.98.
Conclusion: This study shows that serology is a group of tests with high accuracy, mainly following the second week after infection.
Keywords: Diagnostic meta-analysis; SARS-CoV-2; Sensitivity; Specificity; Systematic review.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
The present study has no conflicts of interest.
Figures
References
-
- Johns Hopkins University and medicine. Coronavirus 2019-nCoV, CSSE. Coronavirus 2019-nCoV global cases by Johns Hopkins CSSE. (Available from: https://coronavirus.jhu.edu/map.html). April 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous