Potential for the lung recruitment and the risk of lung overdistension during 21 days of mechanical ventilation in patients with COVID-19 after noninvasive ventilation failure: the COVID-VENT observational trial
- PMID: 35246024
- PMCID: PMC8894841
- DOI: 10.1186/s12871-022-01600-0
Potential for the lung recruitment and the risk of lung overdistension during 21 days of mechanical ventilation in patients with COVID-19 after noninvasive ventilation failure: the COVID-VENT observational trial
Abstract
Background: Data on the lung respiratory mechanics and gas exchange in the time course of COVID-19-associated respiratory failure is limited. This study aimed to explore respiratory mechanics and gas exchange, the lung recruitability and risk of overdistension during the time course of mechanical ventilation.
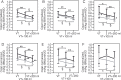
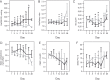
Methods: This was a prospective observational study in critically ill mechanically ventilated patients (n = 116) with COVID-19 admitted into Intensive Care Units of Sechenov University. The primary endpoints were: «optimum» positive end-expiratory pressure (PEEP) level balanced between the lowest driving pressure and the highest SpO2 and number of patients with recruitable lung on Days 1 and 7 of mechanical ventilation. We measured driving pressure at different levels of PEEP (14, 12, 10 and 8 cmH2O) with preset tidal volume, and with the increase of tidal volume by 100 ml and 200 ml at preset PEEP level, and calculated static respiratory system compliance (CRS), PaO2/FiO2, alveolar dead space and ventilatory ratio on Days 1, 3, 5, 7, 10, 14 and 21.
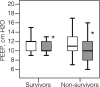
Results: The «optimum» PEEP levels on Day 1 were 11.0 (10.0-12.8) cmH2O and 10.0 (9.0-12.0) cmH2O on Day 7. Positive response to recruitment was observed on Day 1 in 27.6% and on Day 7 in 9.2% of patients. PEEP increase from 10 to 14 cmH2O and VT increase by 100 and 200 ml led to a significant decrease in CRS from Day 1 to Day 14 (p < 0.05). Ventilatory ratio was 2.2 (1.7-2,7) in non-survivors and in 1.9 (1.6-2.6) survivors on Day 1 and decreased on Day 7 in survivors only (p < 0.01). PaO2/FiO2 was 105.5 (76.2-141.7) mmHg in non-survivors on Day 1 and 136.6 (106.7-160.8) in survivors (p = 0.002). In survivors, PaO2/FiO2 rose on Day 3 (p = 0.008) and then between Days 7 and 10 (p = 0.046).
Conclusion: Lung recruitability was low in COVID-19 and decreased during the course of the disease, but lung overdistension occurred at «intermediate» PEEP and VT levels. In survivors gas exchange improvements after Day 7 mismatched CRS.
Trial registration: ClinicalTrials.gov, NCT04445961 . Registered 24 June 2020-Retrospectively registered.
Keywords: Acute respiratory distress syndrome; COVID-19; Lung recruitability; Lung strain; PEEP; Volutrauma.
© 2022. The Author(s).
Conflict of interest statement
AIY reported personal fees from GE, Philips Respironics, Covidien, Fisher & Paykel, Drager, Triton Electronics, Mindray, Pfizer, BBraun, Gilead outside the submitted work. SNA reported personal fees from Behringer Ingelheim, Pfizer, Novartis, AstraZeneca, Chiesi outside the submitted work. No other disclosers were reported.
Figures


Similar articles
-
Recruitability and effect of PEEP in SARS-Cov-2-associated acute respiratory distress syndrome.Ann Intensive Care. 2020 May 12;10(1):55. doi: 10.1186/s13613-020-00675-7. Ann Intensive Care. 2020. PMID: 32399901 Free PMC article.
-
Positive end-expiratory pressure in COVID-19 acute respiratory distress syndrome: the heterogeneous effects.Crit Care. 2021 Dec 16;25(1):431. doi: 10.1186/s13054-021-03839-4. Crit Care. 2021. PMID: 34915911 Free PMC article.
-
Correlation between normally aerated lung and respiratory system compliance at clinical high positive end-expiratory pressure in patients with COVID-19.Sci Rep. 2024 Jun 24;14(1):14477. doi: 10.1038/s41598-024-64622-3. Sci Rep. 2024. PMID: 38914620 Free PMC article.
-
Mechanical ventilation in COVID-19: A physiological perspective.Exp Physiol. 2022 Jul;107(7):683-693. doi: 10.1113/EP089400. Epub 2021 Sep 27. Exp Physiol. 2022. PMID: 34541721 Free PMC article. Review.
-
Mechanical ventilation parameters in critically ill COVID-19 patients: a scoping review.Crit Care. 2021 Mar 20;25(1):115. doi: 10.1186/s13054-021-03536-2. Crit Care. 2021. PMID: 33743812 Free PMC article.
Cited by
-
Effect of flow-optimized pressure control ventilation-volume guaranteed (PCV-VG) on postoperative pulmonary complications: a consort study.J Cardiothorac Surg. 2024 Jul 8;19(1):425. doi: 10.1186/s13019-024-02881-x. J Cardiothorac Surg. 2024. PMID: 38978064 Free PMC article. Clinical Trial.
-
Determination of positive end-expiratory pressure in COVID-19-related acute respiratory distress syndrome: A systematic review.Eur J Anaesthesiol Intensive Care. 2024 Oct 4;3(6):e0060. doi: 10.1097/EA9.0000000000000060. eCollection 2024 Dec. Eur J Anaesthesiol Intensive Care. 2024. PMID: 39917636 Free PMC article.
-
Breathing pattern, accessory respiratory muscles work, and gas exchange evaluation for prediction of NIV failure in moderate-to-severe COVID-19-associated ARDS after deterioration of respiratory failure outside ICU: the COVID-NIV observational study.BMC Anesthesiol. 2022 Oct 1;22(1):307. doi: 10.1186/s12871-022-01847-7. BMC Anesthesiol. 2022. PMID: 36183064 Free PMC article.
References
-
- Diehl JL, Peron N, Chocron R, Debuc B, Guerot E, Hauw-Berlemont C, Hermann B, Augy JL, Younan R, Novara A, Langlais J, Khider L, Gendron N, Goudot G, Fagon JF, Mirault T, Smadja DM. Respiratory mechanics and gas exchanges in the early course of COVID-19 ARDS: a hypothesis-generating study. Ann Intensive Care. 2020;10:95. doi: 10.1186/s13613-020-00716-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

