An international comparative analysis of public reimbursement of orphan drugs in Canadian provinces compared to European countries
- PMID: 35246200
- PMCID: PMC8895096
- DOI: 10.1186/s13023-022-02260-6
An international comparative analysis of public reimbursement of orphan drugs in Canadian provinces compared to European countries
Abstract
Background: The Canadian government has committed to developing a national strategy for drugs for rare diseases starting in 2022. Considering this announcement, we conducted a comparative analysis to examine patient access to therapies for rare disease in Canada relative to Europe and the U.S.
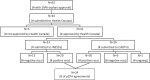
Methods: Given its similarity to the Canadian health care system, we used Europe as the reference point to analyze all of the therapies with an orphan drug designation approved by the European Medicine Agency (EMA) from 1 January 2015 to 31 March 2020. We then contrasted access to these drugs in Canada (Health Canada) and the U.S. (Food and Drug Administration, FDA). We focused on: (1) the number of therapies for rare diseases entering the Canadian market; (2) the percentage of these therapies that are publicly available to Canadians; and (3) the timelines for patients to access these therapies in Canada.
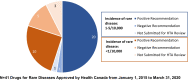
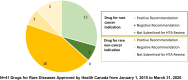
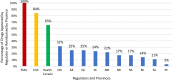
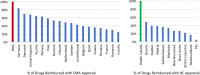
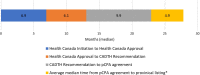
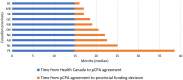
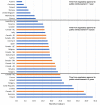
Results: Sixty-three approved therapies with an orphan drug designation from the EMA were identified. Fifty-three (84%) of these drugs had also been submitted to the FDA for approval, and 41 (65%) were submitted to Health Canada for approval. In Europe, Germany, Denmark, and the U.K. had the highest percentage of publicly reimbursed orphan drugs (84%, 70%, 68%, respectively). In comparison, Ontario (32%), Quebec (25%), and Alberta (25%) had the highest percentage of drugs reimbursed among the Canadian provinces. The shortest median duration (in months) from EMA approval to jurisdictional decision on reimbursement was in Austria (3.2), followed by Germany (4.1), and Finland (6.0). In Canada, the shortest median duration (in months) from regulatory approval to reimbursement was in British Columbia (17.3), Quebec (19.6) and Manitoba (19.6), while the longest duration was in P.E.I (38.5), followed by Nova Scotia (25.9), and Newfoundland (25.1).
Conclusions: Our comparative analysis found that relative to the EU Canadians had less frequent and timely access to therapies for rare diseases. This highlights the need for a rare disease strategy in Canada that allows for clear identification and transparent tracking of the pathway for rare disease drugs, and ultimately optimizes the number of patients with access to these therapies.
Keywords: Funding decisions; Orphan drugs; Patient access; Rare disease; Regulatory approval; Reimbursement.
© 2022. The Author(s).
Conflict of interest statement
LMW has participated in clinical trials with, and/or been a consultant to, PTC, ReveraGen, Catabasis, Amgen, Novartis, Alexion, Ascendis, Ipsen, and Ultragenyx, with funds to LMW’s institution. AC and EM are employed by Novartis Pharmaceuticals.
Figures


References
-
- Health Canada. Canada Health Act. Canada.ca. 2004. https://www.canada.ca/en/health-canada/services/health-care-system/canad....
-
- Impact HTA. Impact-hta.eu. https://www.impact-hta.eu/country-vignettes.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

