The glucose-lowering effect of low-dose diacerein and its responsiveness metabolic markers in uncontrolled diabetes
- PMID: 35246243
- PMCID: PMC8896078
- DOI: 10.1186/s13104-022-05974-9
The glucose-lowering effect of low-dose diacerein and its responsiveness metabolic markers in uncontrolled diabetes
Abstract
Objective: Diacerein inhibits the synthesis and activity of pro-inflammatory cytokines, decreases macrophage infiltration in adipose tissue and thus increases insulin sensitivity and signalling. We conducted this study to determine the efficacy of low-dose diacerein in improving glycaemic control in type 2 diabetes mellitus (T2DM) patients with inadequate glycaemic control and to identify the metabolic determinants for such improvement. We randomised 25 T2DM patients with poor glycaemic control, despite being treated with at least three glucose-lowering agents, to receive diacerein 50 mg once-daily (n = 18) or placebo (n = 17) for 12 weeks. Changes in glycated haemoglobin (HbA1c) were evaluated at the 4th and 12th weeks. Metabolic profiling was performed using liquid chromatography electrospray ionisation quadrupole time-of-flight mass spectrometry.
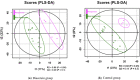
Results: HbA1c levels were significantly reduced from baseline in the diacerein group at 12 weeks (- 0.6%, p < 0.05), whereas fasting plasma glucose (FPG) levels were not significantly decreased (- 18.9 mg/dl, p = 0.06). Partial least squares-discriminant analysis demonstrated an association between the serum abundance of threo-isocitric acid (ICA) and HbA1c response in the diacerein group. After adjusting for serum high-sensitivity C-reactive protein, ICA was still significantly related to the change in HbA1c. Retrospective trial registration Current Controlled Trials TCTR20200820004, 20 August 2020.
Keywords: Diacerein; Glycemic control; Inflammation; Metabolomics; Type 2 diabetes.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Bartels EM, Bliddal H, Schøndorff PK, Altman RD, Zhang W, Christensen R. Symptomatic efficacy and safety of diacerein in the treatment of osteoarthritis: a meta-analysis of randomized placebo-controlled trials. Osteoarthritis Cartilage. 2010;18:289–296. doi: 10.1016/j.joca.2009.10.006. - DOI - PubMed
-
- Ramos-Zavala MG, González-Ortiz M, Martínez-Abundis E, Robles-Cervantes JA, González-López R, Santiago-Hernández NJ. Effect of diacerein on insulin secretion and metabolic control in drug-naive patients with type 2 diabetes: a randomized clinical trial. Diabetes Care. 2011;34:1591–1594. doi: 10.2337/dc11-0357. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous