Immunogenicity of ChAdOx1 nCoV-19 vaccine after a two-dose inactivated SARS-CoV-2 vaccination of dialysis patients and kidney transplant recipients
- PMID: 35246578
- PMCID: PMC8897448
- DOI: 10.1038/s41598-022-07574-w
Immunogenicity of ChAdOx1 nCoV-19 vaccine after a two-dose inactivated SARS-CoV-2 vaccination of dialysis patients and kidney transplant recipients
Abstract
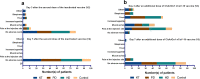
Vaccination with inactivated SARS-CoV-2 virus produces suboptimal immune responses among kidney transplant (KT), peritoneal dialyzed (PD), and hemodialyzed (HD) patients. Participants were vaccinated with two-dose inactivated SARS-CoV-2 vaccine (V2) and a third dose of ChAdOx1 nCoV-19 vaccine (V3) at 1-2 months after V2. We enrolled 106 participants: 31 KT, 28 PD, and 31 HD patients and 16 controls. Among KT, PD, and HD groups, median (IQR) of anti-receptor binding domain antibody levels were 1.0 (0.4-26.8), 1092.5 (606.9-1927.2), and 1740.9 (1106-3762.3) BAU/mL, and percent neutralization was 0.9 (0-9.9), 98.8 (95.9-99.5), and 99.4 (98.8-99.7), respectively, at two weeks after V3. Both parameters were significantly increased from V2 across all groups (p < 0.05). Seroconversion and neutralization positivity rates in PD, HD, and control groups were 100% but were impaired in KT patients (39% and 16%, respectively). S1-specific T-cell counts were increased in PD and HD groups (p < 0.05) but not in KT patients. The positive S1-specific T-cell responder rate was > 90% in PD, HD, and control groups, which was higher than that in KT recipients (74%, p < 0.05). The heterologous inactivated virus/ChAdOx1 nCoV-19 vaccination strategy elicited greater immunogenicity among dialysis patients; however, inadequate responses remained among KT recipients (TCTR20210226002).
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

