Recurrent Subclinical Tuberculosis Among Antiretroviral Therapy-Accessing Participants: Incidence, Clinical Course, and Outcomes
- PMID: 35247054
- PMCID: PMC9617574
- DOI: 10.1093/cid/ciac185
Recurrent Subclinical Tuberculosis Among Antiretroviral Therapy-Accessing Participants: Incidence, Clinical Course, and Outcomes
Abstract
Background: Undiagnosed asymptomatic subclinical tuberculosis (TB) remains a significant threat to global TB control, accounting for a substantial proportion of cases among people living with human immunodeficiency virus (HIV)/AIDS (PLWHA). We determined incidence, progression, and outcomes of subclinical TB in antiretroviral therapy (ART)-accessing PLWHA with known previous TB in South Africa.
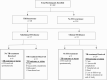
Methods: A total of 402 adult PLWHA previously treated for TB were enrolled in the prospective Centre for the AIDS Programme of Research in South Africa TRuTH (TB Recurrence Upon TB and HIV treatment) Study. Participants were screened for TB with quarterly clinical and bacteriologic evaluation and biannual chest radiographs over 36 months. Those with suspected or confirmed TB were referred to the National TB Programme. Participants received HIV services, including ART. Incidence rate of TB was estimated using Poisson regression and descriptive statistical analyses summarized data.
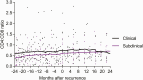
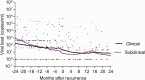
Results: A total of 48 of 402 (11.9%) bacteriologically confirmed incident recurrent TB cases were identified, comprising 17 of 48 (35.4%) subclinical TB cases and 31 of 48 (64.5%) clinical TB cases. Age, sex, and body mass index were similar among subclinical, clinical, and no TB groups. Incidence rates (95% Confidence Interval [CI]) of recurrent TB overall, in clinical and subclinical TB groups were 2.3 (1.7-3.0), 1.5 (1.1-2.2), and 0.9 (0.5-1.4) per 100 person-years, respectively. In the subclinical TB group, 14 of 17 (82.4%) were diagnosed by TB culture only, 11 of 17 (64.7%) received TB treatment, and 6 of 17 (35.3%) resolved TB spontaneously.
Conclusions: High incidence rates of recurrent subclinical TB in PLWHA highlight inadequacies of symptom-based TB screening in high TB-HIV burden settings.
Keywords: HIV; clinical TB; incidence; prevalence; subclinical TB.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. K. N. reports serving on a data and safety monitoring board for the Kharatiwe Study and South African HIV Clinicians Society. H. D. reports honoraria for a lecture from Sanofi South Africa and reports being past president of the Infectious Diseases Society of South Africa. S. S. A. K. reports no payments as a member of the World Health Organization Science Council and as vice-president for the International Science Council and reports meeting honorarium paid to self as a member of the Bill and Melinda Gates Foundation Scientific Advisory Committee. All remaining authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
Similar articles
-
Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control.AIDS. 2006 Aug 1;20(12):1605-12. doi: 10.1097/01.aids.0000238406.93249.cd. AIDS. 2006. PMID: 16868441
-
The shift in tuberculosis timing among people living with HIV in the course of antiretroviral therapy scale-up in Malawi.J Int AIDS Soc. 2019 Apr;22(4):e25240. doi: 10.1002/jia2.25240. J Int AIDS Soc. 2019. PMID: 31038836 Free PMC article.
-
High prevalence of subclinical tuberculosis in HIV-1-infected persons without advanced immunodeficiency: implications for TB screening.Thorax. 2011 Aug;66(8):669-73. doi: 10.1136/thx.2011.160168. Epub 2011 May 31. Thorax. 2011. PMID: 21632522 Free PMC article.
-
Tuberculosis and its association with CD4+ T cell count among adult HIV positive patients in Ethiopian settings: a systematic review and meta-analysis.BMC Infect Dis. 2020 May 7;20(1):325. doi: 10.1186/s12879-020-05040-4. BMC Infect Dis. 2020. PMID: 32380957 Free PMC article.
-
Review of policy and status of implementation of collaborative HIV-TB activities in 23 high-burden countries.Int J Tuberc Lung Dis. 2014 Oct;18(10):1149-58. doi: 10.5588/ijtld.13.0889. Int J Tuberc Lung Dis. 2014. PMID: 25216827 Review.
Cited by
-
The incidence rate of tuberculosis and its associated factors among HIV-positive persons in Sub-Saharan Africa: a systematic review and meta-analysis.BMC Infect Dis. 2023 Sep 18;23(1):613. doi: 10.1186/s12879-023-08533-0. BMC Infect Dis. 2023. PMID: 37723415 Free PMC article.
-
Subclinical tuberculosis linkage to care and completion of treatment following community-based screening in rural South Africa.BMC Glob Public Health. 2024;2(1):30. doi: 10.1186/s44263-024-00059-0. Epub 2024 Jun 2. BMC Glob Public Health. 2024. PMID: 38832047 Free PMC article.
-
Prevalence, Progression, and Treatment of Asymptomatic Tuberculosis: A Prospective Cohort Study in Lanxi County, Zhejiang Province, China.Open Forum Infect Dis. 2025 May 12;12(5):ofaf275. doi: 10.1093/ofid/ofaf275. eCollection 2025 May. Open Forum Infect Dis. 2025. PMID: 40416508 Free PMC article.
-
The burden of subclinical TB in Nigeria.Public Health Action. 2024 Dec 1;14(4):181-185. doi: 10.5588/pha.24.0038. eCollection 2024 Dec. Public Health Action. 2024. PMID: 39618833 Free PMC article.
-
Prevalence of pulmonary tuberculosis and associated factors among adults living with HIV/AIDS in Ethiopia, systematic review and meta-analysis.BMC Infect Dis. 2025 Jan 9;25(1):49. doi: 10.1186/s12879-024-10419-8. BMC Infect Dis. 2025. PMID: 39789440 Free PMC article.
References
-
- World Health Organization. World Health Organization Global Tuberculosis Report 2019. World Health Organization: Geneva, Switzerland. 2019. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed 9 July 2021.
-
- World Health Organization. Systematic Screening for Active Tuberculosis: An Operational Guide. World Health Organization: Geneva, Switzerland. 2015. Available at: http://apps.who.int/iris/bitstream/handle/10665/181164/9789241549172_eng.... Accessed 9 July 2021.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

