Senescent macrophages in the human adipose tissue as a source of inflammaging
- PMID: 35247131
- PMCID: PMC9616990
- DOI: 10.1007/s11357-022-00536-0
Senescent macrophages in the human adipose tissue as a source of inflammaging
Abstract
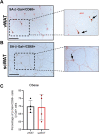
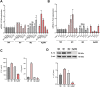
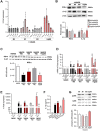
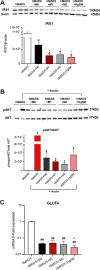
Obesity is a major risk factor for type 2 diabetes and a trigger of chronic and systemic inflammation. Recent evidence suggests that an increased burden of senescent cells (SCs) in the adipose tissue of obese/diabetic animal models might underlie such pro-inflammatory phenotype. However, the role of macrophages as candidate SCs, their phenotype, the distribution of SCs among fat depots, and clinical relevance are debated. The senescence marker β-galactosidase and the macrophage marker CD68 were scored in visceral (vWAT) and subcutaneous (scWAT) adipose tissue from obese patients (n=17) undergoing bariatric surgery and control patients (n=4) subjected to cholecystectomy. A correlation was made between the number of SCs and BMI, serum insulin, and the insulin resistance (IR) index HOMA. The monocyte cell line (THP-1) was cultured in vitro in high glucose milieu (60 mM D-glucose) and subsequently co-cultured with human adipocytes (hMADS) to investigate the reciprocal inflammatory activation. In obese patients, a significantly higher number of SCs was observed in vWAT compared to scWAT; about 70% of these cells expressed the macrophage marker CD68; and the number of SCs in vWAT, but not in scWAT, positively correlated with BMI, HOMA-IR, and insulin. THP-1 cultured in vitro in high glucose milieu acquired a senescent-like phenotype (HgSMs), characterized by a polarization toward a mixed M1/M2-like secretory phenotype. Co-culturing HgSMs with hMADS elicited pro-inflammatory cytokine expression in both cell types, and defective insulin signaling in hMADS. In morbid obesity, expansion of visceral adipose depots involves an increased burden of macrophages with senescent-like phenotype that may promote a pro-inflammatory profile and impair insulin signaling in adipocytes, supporting a framework where senescent macrophages fuel obesity-induced systemic inflammation and possibly contribute to the development of IR.
Keywords: Adipose tissue; Inflammaging; Insulin resistance; Macrophage; Obesity; Senescent cell.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Franceschi, C., et al., Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. - PubMed
-
- Fulop T, et al. The integration of inflammaging in age-related diseases. Semin Immunol. 2018;40:17–35. - PubMed
-
- Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13(11):633–643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

