Comparative Outcomes of Commonly Used Off-Label Atypical Antipsychotics in the Treatment of Dementia-Related Psychosis: A Network Meta-analysis
- PMID: 35247186
- PMCID: PMC9056477
- DOI: 10.1007/s12325-022-02075-8
Comparative Outcomes of Commonly Used Off-Label Atypical Antipsychotics in the Treatment of Dementia-Related Psychosis: A Network Meta-analysis
Abstract
Introduction: Dementia-related psychosis (DRP) is characterized by hallucinations and delusions, which may increase the debilitating effects of underlying dementia. This network meta-analysis (NMA) evaluated the comparative efficacy, safety, and acceptability of atypical antipsychotics (AAPs) commonly used off label to treat DRP.
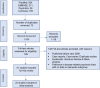
Methods: We included 22 eligible studies from a systematic literature review of AAPs (quetiapine, risperidone, olanzapine, aripiprazole, and brexpiprazole) used off label to treat DRP. Study outcomes were: (1) efficacy-neuropsychiatric inventory-nursing home (NPI-NH psychosis subscale), (2) safety-mortality, cerebrovascular events (CVAEs), and others (somnolence, falls, fractures, injuries, etc.), and (3) acceptability-discontinuations due to all causes, lack of efficacy, and adverse events (AEs). We used random-effects modeling to estimate pooled standardized mean differences (SMDs) for NPI-NH psychosis subscale scores and odds ratios (OR) for other dichotomous outcomes, with their respective 95% confidence intervals (CIs).
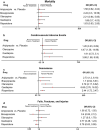
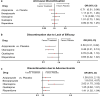
Results: Compared with placebo, aripiprazole (SMD - 0.12; 95% CI - 0.31, 0.06), and olanzapine (SMD - 0.17; 95% CI - 0.04; 0.02) demonstrated small, non-significant numerical improvements in NPI-NH psychosis scores (5 studies; n = 1891), while quetiapine (SMD 0.04; 95% CI - 0.23, 0.32) did not improve symptoms. The odds of mortality (15 studies, n = 4989) were higher for aripiprazole (OR 1.58; 95% CI 0.62, 4.04), brexpiprazole (OR 2.22; 95% CI 0.30, 16.56), olanzapine (OR 2.21; 95% CI 0.84, 5.85), quetiapine (OR 1.68; 95% CI 0.70, 4.03), and risperidone (OR 1.63; 95% CI 0.93, 2.85) than for placebo. Risperidone (OR 3.68; 95% CI 1.68, 8.95) and olanzapine (OR 4.47; 95% CI 1.36, 14.69) demonstrated significantly greater odds of CVAEs compared to placebo. Compared with placebo, odds of all-cause discontinuation were significantly lower for aripiprazole (OR 0.71; 95% CI 0.51, 0.98; 20 studies; 5744 patients) and higher for other AAPs. Aripiprazole (OR 0.5; 95% CI 0.31, 0.82) and olanzapine (OR 0.48; 95% CI 0.31, 0.74) had significantly lower odds of discontinuation due to lack of efficacy (OR 12 studies; n = 4382) compared to placebo, while results for quetiapine and risperidone were not significant. Compared with placebo, the odds of discontinuation due to AEs (19 studies, n = 5445) were higher for olanzapine (OR 2.62; 95% CI 1.75, 3.92), brexpiprazole (OR 1.80; 95% CI 0.80, 4.07), quetiapine (OR 1.25; 95% CI 0.82, 1.91), aripiprazole (OR 1.38; 95% CI 0.90, 2.13), and risperidone (OR 1.41; 95% CI 1.02, 1.94).
Conclusions: Overall results demonstrate that, compared with placebo, quetiapine is not associated with improvement in psychosis in patients with dementia, while olanzapine and aripiprazole have non-significant small numerical improvements. These off-label AAPs (quetiapine, risperidone, olanzapine, aripiprazole, and brexpiprazole) are associated with greater odds of mortality, CVAEs, and discontinuations due to AEs than placebo. These results underscore the ongoing unmet need for newer pharmacological options with a more favorable benefit-risk profile for the treatment of DRP.
Keywords: Acceptability; Atypical antipsychotics; Delusions; Dementia-related psychosis; Efficacy; Hallucination; Network meta-analysis; Safety.
© 2022. The Author(s).
Figures

References
-
- Small GW. Managing the burden of dementia related delusions and hallucinations. J Fam Pract. 2020;69(7 Suppl):S39–S44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

