Efficacy of BCG Vaccination Against Respiratory Tract Infections in Older Adults During the Coronavirus Disease 2019 Pandemic
- PMID: 35247264
- PMCID: PMC8903481
- DOI: 10.1093/cid/ciac182
Efficacy of BCG Vaccination Against Respiratory Tract Infections in Older Adults During the Coronavirus Disease 2019 Pandemic
Abstract
Background: Older age is associated with increased severity and death from respiratory infections, including coronavirus disease 2019 (COVID-19). The tuberculosis BCG vaccine may provide heterologous protection against nontuberculous infections and has been proposed as a potential preventive strategy against COVID-19.
Methods: In this multicenter, placebo-controlled trial, we randomly assigned older adults (aged ≥60 years; n = 2014) to intracutaneous vaccination with BCG vaccine (n = 1008) or placebo (n = 1006). The primary end point was the cumulative incidence of respiratory tract infections (RTIs) that required medical intervention, during 12 months of follow-up. Secondary end points included the incidence of COVID-19, and the effect of BCG vaccination on the cellular and humoral immune responses.
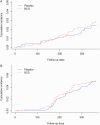
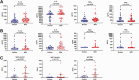
Results: The cumulative incidence of RTIs requiring medical intervention was 0.029 in the BCG-vaccinated group and 0.024 in the control group (subdistribution hazard ratio, 1.26 [98.2% confidence interval, .65-2.44]). In the BCG vaccine and placebo groups, 51 and 48 individuals, respectively tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with polymerase chain reaction (subdistribution hazard ratio, 1.053 [95% confidence interval, .71-1.56]). No difference was observed in the frequency of adverse events. BCG vaccination was associated with enhanced cytokine responses after influenza, and also partially associated after SARS-CoV-2 stimulation. In patients diagnosed with COVID-19, antibody responses after infection were significantly stronger if the volunteers had previously received BCG vaccine.
Conclusions: BCG vaccination had no effect on the incidence of RTIs, including SARS-CoV-2 infection, in older adult volunteers. However, it improved cytokine responses stimulated by influenza and SARS-CoV-2 and induced stronger antibody titers after COVID-19 infection.
Clinical trials registration: EU Clinical Trials Register 2020-001591-15 ClinicalTrials.gov NCT04417335.
Keywords: BCG vaccination; COVID-19; SARS-CoV-2; trained immunity.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- Pera A, Campos C, López N, et al. . Immunosenescence: implications for response to infection and vaccination in older people. Maturitas 2015; 82:50–5. - PubMed
-
- Moorlag S, Arts RJW, van Crevel R, Netea MG.. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect 2019; 25:1473–8. - PubMed
-
- Aaby P, Roth A, Ravn H, Napirna BM, Rodrigues A, Lisse IM.. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis 2011; 204:245–52. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

