DNA aptamer selection for SARS-CoV-2 spike glycoprotein detection
- PMID: 35247355
- PMCID: PMC8889740
- DOI: 10.1016/j.ab.2022.114633
DNA aptamer selection for SARS-CoV-2 spike glycoprotein detection
Abstract
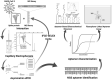
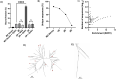
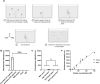
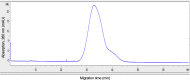
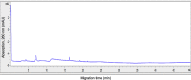
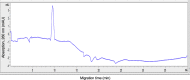
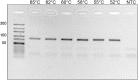
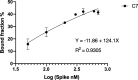
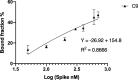
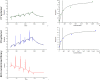
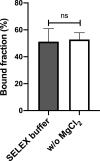
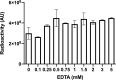
The rapid spread of SARS-CoV-2 infection throughout the world led to a global public health and economic crisis triggering an urgent need for the development of low-cost vaccines, therapies and high-throughput detection assays. In this work, we used a combination of Ideal-Filter Capillary Electrophoresis SELEX (IFCE-SELEX), Next Generation Sequencing (NGS) and binding assays to isolate and validate single-stranded DNA aptamers that can specifically recognize the SARS-CoV-2 Spike glycoprotein. Two selected non-competing DNA aptamers, C7 and C9 were successfully used as sensitive and specific biological recognition elements for the development of electrochemical and fluorescent aptasensors for the SARS-CoV-2 Spike glycoprotein with detection limits of 0.07 fM and 41.87 nM, respectively.
Keywords: Aptamers; Aptasensor; COVID-19; Capillary electrophoresis; SARS-CoV-2; SELEX.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Ultra-Sensitive Aptamer-Based Diagnostic Systems for Rapid Detection of All SARS-CoV-2 Variants.Int J Mol Sci. 2025 Jan 16;26(2):745. doi: 10.3390/ijms26020745. Int J Mol Sci. 2025. PMID: 39859459 Free PMC article.
-
Diverse high-affinity DNA aptamers for wild-type and B.1.1.7 SARS-CoV-2 spike proteins from a pre-structured DNA library.Nucleic Acids Res. 2021 Jul 21;49(13):7267-7279. doi: 10.1093/nar/gkab574. Nucleic Acids Res. 2021. PMID: 34232998 Free PMC article.
-
In vitro selection of a single-strand DNA aptamer targeting the receptor-binding domain of SARS-CoV-2 spike protein.Nucleosides Nucleotides Nucleic Acids. 2023;42(2):105-118. doi: 10.1080/15257770.2022.2109170. Epub 2022 Aug 10. Nucleosides Nucleotides Nucleic Acids. 2023. PMID: 35949145
-
Aptamers-Diagnostic and Therapeutic Solution in SARS-CoV-2.Int J Mol Sci. 2022 Jan 26;23(3):1412. doi: 10.3390/ijms23031412. Int J Mol Sci. 2022. PMID: 35163338 Free PMC article. Review.
-
In silico selection of aptamers against SARS-CoV-2.Analyst. 2024 Sep 23;149(19):4770-4788. doi: 10.1039/d4an00812j. Analyst. 2024. PMID: 39221970 Review.
Cited by
-
Fluorescence-Linked Aptamer Assay for SARS-CoV-2 Spike-Protein: A Step-by-Step Performance Analysis in Clinical Samples.Diagnostics (Basel). 2022 Nov 17;12(11):2829. doi: 10.3390/diagnostics12112829. Diagnostics (Basel). 2022. PMID: 36428893 Free PMC article.
-
Aptamers and Nanobodies as New Bioprobes for SARS-CoV-2 Diagnostic and Therapeutic System Applications.Biosensors (Basel). 2024 Mar 15;14(3):146. doi: 10.3390/bios14030146. Biosensors (Basel). 2024. PMID: 38534253 Free PMC article. Review.
-
A Truncated Multi-Thiol Aptamer-Based SARS-CoV-2 Electrochemical Biosensor: Towards Variant-Specific Point-of-Care Detection with Optimized Fabrication.Biosensors (Basel). 2025 Jan 6;15(1):24. doi: 10.3390/bios15010024. Biosensors (Basel). 2025. PMID: 39852074 Free PMC article.
-
Aptamers Targeting Membrane Proteins for Sensor and Diagnostic Applications.Molecules. 2023 Apr 26;28(9):3728. doi: 10.3390/molecules28093728. Molecules. 2023. PMID: 37175137 Free PMC article. Review.
-
Quantitative Characterization of Partitioning Stringency in SELEX.JACS Au. 2024 Dec 9;4(12):4910-4920. doi: 10.1021/jacsau.4c00890. eCollection 2024 Dec 23. JACS Au. 2024. PMID: 39735910 Free PMC article.
References
-
- Hui D.S., I Azhar E., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., Mchugh T.D., Memish Z.A., Drosten C., Zumla A., Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. - DOI - PMC - PubMed
-
- WHO . 1–23. World Heal. Organ; 2021. https://www.who.int/publications/m/item/covid-19-weekly-epidemiological-... (COVID-19 Weekly Epidemiological Update).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

