Lung damage created by high tidal volume ventilation in rats with monocrotaline-induced pulmonary hypertension
- PMID: 35247989
- PMCID: PMC8897872
- DOI: 10.1186/s12890-022-01867-6
Lung damage created by high tidal volume ventilation in rats with monocrotaline-induced pulmonary hypertension
Abstract
Background: Rats with chronic hypoxia-induced non-inflammatory pulmonary hypertension (PH) are resistant to ventilator-induced lung injury. We investigated the effect of high tidal volume ventilation in another model of PH, monocrotaline (MCT)-induced PH, which is a type of inflammatory PH.
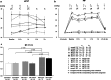
Methods: PH was induced in rats by subcutaneous injection with 60 mg/kg MCT. Normal control rats, rats at 2 weeks after MCT injection (MCT2), and rats at 3 weeks after MCT injection (MCT3) were ventilated with low tidal volume (LV, 6 mL/kg) or high tidal volume (HV, 35 mL/kg) for 2 h with room air without positive end-expiratory pressure. Arterial oxygen pressure (PaO2) and Evans blue dye (EBD) extravasation were measured. Hypertensive pulmonary vascular remodeling was assessed morphometrically by the percentage of muscularized peripheral pulmonary arteries (%Muscularization) and the media wall thickness to external diameter ratio, namely percentage medial wall thickness (%MWT). To assess inflammation, lung IκB protein and cytokine mRNA expression levels were assessed.
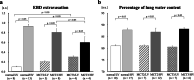
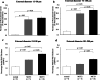
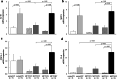
Results: Baseline mean pulmonary arterial pressure was significantly higher in MCT rats (normal, 15.4 ± 0.5 mmHg; MCT2, 23.7 ± 0.9; and MCT3, 34.5 ± 1.5). After 2-h ventilation, PaO2 was significantly lower in the HV groups compared with the LV groups in normal and MCT2 rats, but not in MCT3 rats. Impairment of oxygenation with HV was less in MCT3 rats compared with normal and MCT2 rats. Among the HV groups, MCT3 rats showed significantly lower levels of EBD extravasation than normal and MCT2 rats. HV significantly downregulated IκB protein expression in normal and MCT3 rats and increased IL-6, MCP-1, CXCL-1 (MIP-1), and IL-10 mRNA levels in MCT3 rats. %Muscularization, %MWT, and the expression of lung elastin were significantly higher in MCT3 rats than in normal and MCT2 rats.
Conclusion: We found that HV-associated damage might be reduced in MCT-induced PH rats compared with normal rats. The results of this and earlier studies suggest that hypertensive pulmonary vascular structural changes might be protective against the occurrence of ventilator-induced lung injury, irrespective of the etiology of PH.
Keywords: Mechanical ventilation; Monocrotaline; Pulmonary hypertension; Ventilator-induced lung injury.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Curley GF, Laffey JG, Zhang H, Slutsky AS. Biotrauma and ventilator-induced lung injury: clinical implications. Chest. 2016;150(5):1109–1117. - PubMed
-
- Tremblay LN, Slutsky AS. Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med. 2006;32(1):24–33. - PubMed
-
- Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2014;370(10):980. - PubMed
-
- Dreyfuss D, Soler P, Saumon G. Mechanical ventilation-induced pulmonary edema. Interaction with previous lung alterations. Am J Respir Crit Care Med. 1995;151(5):1568–1575. - PubMed
-
- Chiumello D, Pristine G, Slutsky AS. Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160(1):109–116. - PubMed
MeSH terms
Substances
Grants and funding
- 18K08192/The Japanese Ministry of Education, Culture, Sports, Science and Technology
- 18K16464/The Japanese Ministry of Education, Culture, Sports, Science and Technology.
- 16K10933/The Japanese Ministry of Education, Culture, Sports, Science and Technology.
- 17K10140/The Japanese Ministry of Education, Culture, Sports, Science and Technology.
- 17K11075/The Japanese Ministry of Education, Culture, Sports, Science and Technology.
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

