Adverse effect investigation using application software after vaccination against SARS-CoV-2 for healthcare workers
- PMID: 35248497
- PMCID: PMC8885303
- DOI: 10.1016/j.jiac.2022.02.020
Adverse effect investigation using application software after vaccination against SARS-CoV-2 for healthcare workers
Abstract
Introduction: The usefulness of smartphone-based application software as a way to manage adverse events (AEs) after vaccination is well known. The purpose of this study is to clarify the usefulness and precautions of employing a smartphone application for collecting AEs after the administration of Comirnaty®️.
Methods: Healthcare workers (HCWs) who were vaccinated with Comirnaty®️ were asked to register for the application software and to report AEs for 14 days after vaccination. AEs were self-reported according to severity. The software was set to output an alert in case of fever.
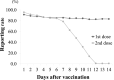
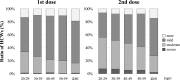
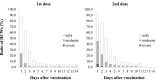
Results: The number of HCWs who received the first dose was 2,551, and 2,406 (94.3%) reported their vaccinations. 2,547 received the second dose, and 2,347 (92.1%) reported their vaccinations. With the first dose, the reporting rate stayed above 83.3% until the final day. On the other hand, that of the second dose decreased rapidly after 6 days. The most frequent symptom was "pain at injection site" (more than 70%). Severe AEs were 6.6% after the second dose, with 0.6% visiting a clinic. Many AEs peaked on the day after administration and disappeared within 1 week. There were few reports of fever.
Conclusion: Smartphone applications can be used to collect information on AEs after vaccination. Application settings and dissemination are necessary to maintain the reporting rate of HCWs.
Keywords: Adverse event; Application software; SARS-CoV2; Vaccine.
Copyright © 2022 Japanese Society of Chemotherapy and The Japanese Association for Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Taka-aki Nakada (TN) is an inventor and has submitted patents related to respon:sum system. TN serves as CEO, receives executive compensation and holds shares in Smart119 Inc., which develops the respon:sum system.
Figures
Similar articles
-
Self-Reported adverse events among Chinese healthcare workers immunized with COVID-19 vaccines composed of inactivated SARS-CoV-2.Hum Vaccin Immunother. 2022 Nov 30;18(5):2064134. doi: 10.1080/21645515.2022.2064134. Epub 2022 Apr 22. Hum Vaccin Immunother. 2022. PMID: 35452357 Free PMC article.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Adverse reactions to the first and second doses of Pfizer-BioNTech COVID-19 vaccine among healthcare workers.J Infect Chemother. 2022 Jul;28(7):934-942. doi: 10.1016/j.jiac.2022.03.015. Epub 2022 Mar 25. J Infect Chemother. 2022. PMID: 35361536 Free PMC article.
-
Influence of epidemiological and clinical factors in the reactogenicity to Comirnaty® vaccine in health care workers of a Spanish university teaching hospital (COVIVAC study).Rev Esp Quimioter. 2023 Aug;36(4):400-407. doi: 10.37201/req/017.2023. Epub 2023 Apr 29. Rev Esp Quimioter. 2023. PMID: 37119130 Free PMC article.
-
Facial Dermal Filler Injection and Vaccination: A 12-Year Review of Adverse Event Reporting and Literature Review.Aesthet Surg J. 2023 Jun 14;43(7):NP544-NP557. doi: 10.1093/asj/sjad031. Aesthet Surg J. 2023. PMID: 36788718 Review.
Cited by
-
A scoping review of active, participant centred, digital adverse events following immunization (AEFI) surveillance of WHO approved COVID-19 vaccines: A Canadian immunization Research Network study.Hum Vaccin Immunother. 2024 Dec 31;20(1):2293550. doi: 10.1080/21645515.2023.2293550. Epub 2024 Feb 19. Hum Vaccin Immunother. 2024. PMID: 38374618 Free PMC article.
-
Applications of Social Media and Digital Technologies in COVID-19 Vaccination: Scoping Review.J Med Internet Res. 2023 Feb 10;25:e40057. doi: 10.2196/40057. J Med Internet Res. 2023. PMID: 36649235 Free PMC article.
References
-
- Fabiani M., Ramigni M., Gobbetto V., Mateo-Urdiales A., Pezzotti P., Piovesan C. Vol. 26. 2021. p. 2100420. (Euro Surveill. Effectiveness of the Comirnaty (BNT162b2, BioNTech/Pfizer) vaccine in preventing SARS-CoV-2 infection among healthcare workers, Treviso province, Veneto region). Italy, 27 December 2020 to 24 March 2021. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

