Randomized Controlled Trial Examining the Efficacy of Adding Financial Incentives to Best practices for Smoking Cessation Among pregnant and Newly postpartum Women
- PMID: 35248683
- PMCID: PMC9440164
- DOI: 10.1016/j.ypmed.2022.107012
Randomized Controlled Trial Examining the Efficacy of Adding Financial Incentives to Best practices for Smoking Cessation Among pregnant and Newly postpartum Women
Abstract
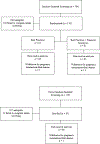
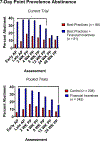
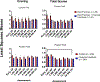
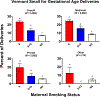
We report results from a single-blinded randomized controlled trial examining financial incentives for smoking cessation among 249 pregnant and newly postpartum women. Participants included 169 women assigned to best practices (BP) or BP plus financial incentives (BP + FI) for smoking cessation available through 12-weeks postpartum. A third condition included 80 never-smokers (NS) sociodemographically-matched to women who smoked. Trial setting was Burlington, Vermont, USA, January, 2014 through January, 2020. Outcomes included 7-day point-prevalence abstinence antepartum and postpartum, and birth and other infant outcomes during 1st year of life. Reliability and external validity of results were assessed using pooled results from the current and four prior controlled trials coupled with data on maternal-smoking status and birth outcomes for all 2019 singleton live births in Vermont. Compared to BP, BP + FI significantly increased abstinence early- (AOR = 9.97; 95%CI, 3.32-29.93) and late-pregnancy (primary outcome, AOR = 5.61; 95%CI, 2.37-13.28) and through 12-weeks postpartum (AOR = 2.46; CI,1.05-5.75) although not 24- (AOR = 1.31; CI,0.54-3.17) or 48-weeks postpartum (AOR = 1.33; CI,0.55-3.25). There was a significant effect of trial condition on small-for-gestational-age (SGA) deliveries (χ2 [2] = 9.01, P = .01), with percent SGA deliveries (+SEM) greatest in BP, intermediate in BP + FI, and lowest in NS (17.65 + 4.13, 10.81 + 3.61, and 2.53 + 1.77, respectively). Reliability analyses supported the efficacy of financial incentives for increasing abstinence antepartum and postpartum and decreasing SGA deliveries; external-validity analyses supported relationships between antepartum cessation and SGA risk. Adding financial incentives to Best Practice increases smoking cessation among antepartum and postpartum women and improves other maternal-infant outcomes. TRIAL REGISTRATION: ClinicalTrials.gov Identifier: NCT02210832.
Keywords: Cost-effectiveness; birth outcomes; cigarette smoking; contingency management; financial incentives; pregnancy; small-for-gestational-age delivery; smoking cessation.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors report no conflicts of interest to disclose.
Figures

References
-
- Ages & Stages Questionnaires®, Third Edition. https://agesandstages.com/products-pricing/asq3/; last accessed July 30, 2021.
-
- American College of Obstetricians and Gynecologists. Breastfeeding your baby. https://www.acog.org/womens-health/faqs/breastfeeding-your-baby. Content last updated, May 2021. Site visited September 10, 2021.
-
- Armitage P Exclusions, losses to follow-up, and withdrawals in clinical trials. In Shapiro SH & Lewis TA (Eds.), Clinical trials: Issues and approaches. 1983; pp. 99–113. New York: Marcel Dekker, Inc.
-
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

