Lipid rafts as viral entry routes and immune platforms: A double-edged sword in SARS-CoV-2 infection?
- PMID: 35248801
- PMCID: PMC8894694
- DOI: 10.1016/j.bbalip.2022.159140
Lipid rafts as viral entry routes and immune platforms: A double-edged sword in SARS-CoV-2 infection?
Abstract
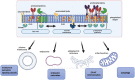
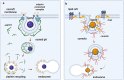
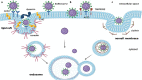
Lipid rafts are nanoscopic compartments of cell membranes that serve a variety of biological functions. They play a crucial role in viral infections, as enveloped viruses such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can exploit rafts to enter or quit target cells. On the other hand, lipid rafts contribute to the formation of immune synapses and their proper functioning is a prerequisite for adequate immune response and viral clearance. In this narrative review we dissect the panorama focusing on this singular aspect of cell biology in the context of SARS-CoV-2 infection and therapy. A lipid raft-mediated mechanism can be hypothesized for many drugs recommended or considered for the treatment of SARS-CoV-2 infection, such as glucocorticoids, antimalarials, immunosuppressants and antiviral agents. Furthermore, the additional use of lipid-lowering agents, like statins, may affect the lipid composition of membrane rafts and thus influence the processes occurring in these compartments. The combination of drugs acting on lipid rafts may be successful in the treatment of more severe forms of the disease and should be reserved for further investigation.
Keywords: Anticoagulant drugs; Antiviral drugs; COVID-19; Immunosuppressive drugs; Lipid rafts; Lipid-lowering drugs; Monoclonal antibodies; SARS-CoV-2.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Cholesterol-Rich Lipid Rafts as Platforms for SARS-CoV-2 Entry.Front Immunol. 2021 Dec 16;12:796855. doi: 10.3389/fimmu.2021.796855. eCollection 2021. Front Immunol. 2021. PMID: 34975904 Free PMC article. Review.
-
Lipid Raft Integrity and Cellular Cholesterol Homeostasis Are Critical for SARS-CoV-2 Entry into Cells.Nutrients. 2022 Aug 19;14(16):3417. doi: 10.3390/nu14163417. Nutrients. 2022. PMID: 36014919 Free PMC article.
-
Lipid rafts are involved in SARS-CoV entry into Vero E6 cells.Biochem Biophys Res Commun. 2008 May 2;369(2):344-9. doi: 10.1016/j.bbrc.2008.02.023. Epub 2008 Feb 13. Biochem Biophys Res Commun. 2008. PMID: 18279660 Free PMC article.
-
Simvastatin Downregulates the SARS-CoV-2-Induced Inflammatory Response and Impairs Viral Infection Through Disruption of Lipid Rafts.Front Immunol. 2022 Feb 18;13:820131. doi: 10.3389/fimmu.2022.820131. eCollection 2022. Front Immunol. 2022. PMID: 35251001 Free PMC article.
-
Coronavirus Interplay With Lipid Rafts and Autophagy Unveils Promising Therapeutic Targets.Front Microbiol. 2020 Aug 11;11:1821. doi: 10.3389/fmicb.2020.01821. eCollection 2020. Front Microbiol. 2020. PMID: 32849425 Free PMC article. Review.
Cited by
-
Virally-initiated pain states: phenotypes, mechanisms, and future directions.Front Pain Res (Lausanne). 2025 Jan 28;6:1527106. doi: 10.3389/fpain.2025.1527106. eCollection 2025. Front Pain Res (Lausanne). 2025. PMID: 39958365 Free PMC article.
-
Lower serum LDL-C levels are associated with poor prognosis in severe fever with thrombocytopenia syndrome: a single-center retrospective cohort study.Front Microbiol. 2024 Jun 24;15:1412263. doi: 10.3389/fmicb.2024.1412263. eCollection 2024. Front Microbiol. 2024. PMID: 38979536 Free PMC article.
-
SARS-CoV-2-associated lymphopenia: possible mechanisms and the role of CD147.Cell Commun Signal. 2024 Jul 4;22(1):349. doi: 10.1186/s12964-024-01718-3. Cell Commun Signal. 2024. PMID: 38965547 Free PMC article. Review.
-
Serum lipidome reveals lipid metabolic dysregulation in severe fever with thrombocytopenia syndrome.BMC Med. 2024 Oct 14;22(1):458. doi: 10.1186/s12916-024-03672-w. BMC Med. 2024. PMID: 39396989 Free PMC article.
-
Research Advances on the Role of Lipids in the Life Cycle of Human Coronaviruses.Microorganisms. 2023 Dec 28;12(1):63. doi: 10.3390/microorganisms12010063. Microorganisms. 2023. PMID: 38257890 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

