The role of endogenous versus exogenous sources in the exposome of putative genotoxins and consequences for risk assessment
- PMID: 35249149
- PMCID: PMC9013691
- DOI: 10.1007/s00204-022-03242-0
The role of endogenous versus exogenous sources in the exposome of putative genotoxins and consequences for risk assessment
Abstract
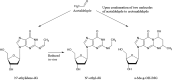
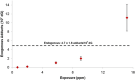
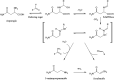
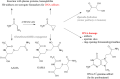
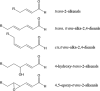
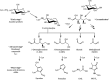
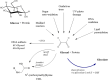
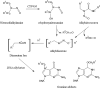
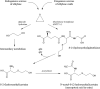
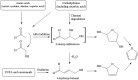
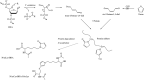
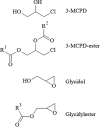
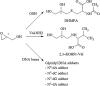
The "totality" of the human exposure is conceived to encompass life-associated endogenous and exogenous aggregate exposures. Process-related contaminants (PRCs) are not only formed in foods by heat processing, but also occur endogenously in the organism as physiological components of energy metabolism, potentially also generated by the human microbiome. To arrive at a comprehensive risk assessment, it is necessary to understand the contribution of in vivo background occurrence as compared to the ingestion from exogenous sources. Hence, this review provides an overview of the knowledge on the contribution of endogenous exposure to the overall exposure to putative genotoxic food contaminants, namely ethanol, acetaldehyde, formaldehyde, acrylamide, acrolein, α,β-unsaturated alkenals, glycation compounds, N-nitroso compounds, ethylene oxide, furans, 2- and 3-MCPD, and glycidyl esters. The evidence discussed herein allows to conclude that endogenous formation of some contaminants appears to contribute substantially to the exposome. This is of critical importance for risk assessment in the cases where endogenous exposure is suspected to outweigh the exogenous one (e.g. formaldehyde and acrolein).
Keywords: Endogenous exposure; Exposome; Genotoxins; Process-related contaminants.
© 2022. The Author(s).
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

