Genetic ablation of the Bsx homeodomain transcription factor in zebrafish: Impact on mature pineal gland morphology and circadian behavior
- PMID: 35249239
- PMCID: PMC9285933
- DOI: 10.1111/jpi.12795
Genetic ablation of the Bsx homeodomain transcription factor in zebrafish: Impact on mature pineal gland morphology and circadian behavior
Abstract
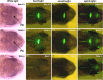
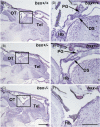
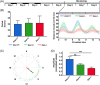
The pineal gland is a neuroendocrine structure in the brain, which produces and secretes the hormone melatonin at nighttime and is considered a key element in the circadian clock system. Early morphogenesis of the gland is controlled by a number of transcription factors, some of which remain active in adult life. One of these is the brain-specific homeobox (Bsx), a highly conserved homeodomain transcription factor with a developmental role in the pineal gland of several species, including zebrafish, and regulatory roles in mature pinealocytes of the rat. To determine the role of Bsx in circadian biology, we here examined the effects of a bsx loss-of-function mutation on the pineal gland in adult zebrafish and on behavioral circadian rhythms in larvae. In pineal cell type-specific Gfp/Egfp reporter zebrafish lines, we did not detect fluorescence signals in the pineal area of homozygous (bsx-/- ) mutants. Interestingly, a nonpigmented area on the dorsal surface of the head above the gland, known as the pineal window, was pigmented in the homozygous mutants. Furthermore, a structure corresponding to the pineal gland was not detectable in the midline of the adult brain in histological sections analyzed by Nissl staining and S-antigen immunohistochemistry. Moreover, the levels of pineal transcripts were greatly reduced in bsx-/- mutants, as revealed by quantitative real-time polymerase chain reaction analysis. Notably, analysis of locomotor activity at the larval stage revealed altered circadian rhythmicity in the bsx mutants with periods and phases similar to wildtype, but severely reduced amplitudes in locomotor activity patterns. Thus, Bsx is essential for full development of the pineal gland, with its absence resulting in a phenotype of morphological pineal gland ablation and disrupted circadian behavior.
Keywords: bsx; circadian; homeobox; locomotor activity assay; loss-of-function; pineal gland; zebrafish.
© 2022 The Authors. Journal of Pineal Research published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Klein DC. Arylalkylamine N‐acetyltransferase: "the Timezyme". J Biol Chem. 2007;282(7):4233‐4237. - PubMed
-
- Cremona M, Colombo E, Andreazzoli M, Cossu G, Broccoli V. Bsx, an evolutionary conserved Brain Specific homeoboX gene expressed in the septum, epiphysis, mammillary bodies and arcuate nucleus. Gene Exp Patterns. 2004;4(1):47‐51. - PubMed
-
- Schredelseker T, Driever W. Bsx controls pineal complex development. Development. 2018;145(13):dev163477. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

