The evolution of sex peptide: sexual conflict, cooperation, and coevolution
- PMID: 35249265
- PMCID: PMC9256762
- DOI: 10.1111/brv.12849
The evolution of sex peptide: sexual conflict, cooperation, and coevolution
Abstract
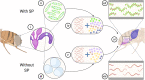
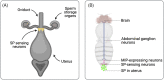
A central paradigm in evolutionary biology is that the fundamental divergence in the fitness interests of the sexes ('sexual conflict') can lead to both the evolution of sex-specific traits that reduce fitness for individuals of the opposite sex, and sexually antagonistic coevolution between the sexes. However, clear examples of traits that evolved in this way - where a single trait in one sex demonstrably depresses the fitness of members of the opposite sex, resulting in antagonistic coevolution - are rare. The Drosophila seminal protein 'sex peptide' (SP) is perhaps the most widely cited example of a trait that appears to harm females while benefitting males. Transferred in the ejaculate by males during mating, SP triggers profound and wide-ranging changes in female behaviour and physiology. Early studies reported that the transfer of SP enhances male fitness while depressing female fitness, providing the foundations for the widespread view that SP has evolved to manipulate females for male benefit. Here, we argue that this view is (i) a simplification of a wider body of contradictory empirical research, (ii) narrow with respect to theory describing the origin and maintenance of sexually selected traits, and (iii) hard to reconcile with what we know of the evolutionary history of SP's effects on females. We begin by charting the history of thought regarding SP, both at proximate (its production, function, and mechanism of action) and ultimate (its fitness consequences and evolutionary history) levels, reviewing how studies of SP were central to the development of the field of sexual conflict. We describe a prevailing paradigm for SP's evolution: that SP originated and continues to evolve to manipulate females for male benefit. In contrast to this view, we argue on three grounds that the weight of evidence does not support the view that receipt of SP decreases female fitness: (i) results from studies of SP's impact on female fitness are mixed and more often neutral or positive, with fitness costs emerging only under nutritional extremes; (ii) whether costs from SP are appreciable in wild-living populations remains untested; and (iii) recently described confounds in genetic manipulations of SP raise the possibility that measures of the costs and benefits of SP have been distorted. Beyond SP's fitness effects, comparative and genetic data are also difficult to square with the idea that females suffer fitness costs from SP. Instead, these data - from functional and evolutionary genetics and the neural circuitry of female responses to SP - suggest an evolutionary history involving the evolution of a dedicated SP-sensing apparatus in the female reproductive tract that is likely to have evolved because it benefits females, rather than harms them. We end by exploring theory and evidence that SP benefits females by functioning as a signal of male quality or of sperm receipt and storage (or both). The expanded view of the evolution of SP that we outline recognises the context-dependent and fluctuating roles played by both cooperative and antagonistic selection in the origin and maintenance of reproductive traits.
Keywords: coevolution; condition dependence; ejaculates; post-mating responses; seminal fluid; sex peptide; sexual conflict; sexual selection; signalling; sperm competition.
© 2022 The Authors. Biological Reviews published by John Wiley & Sons Ltd on behalf of Cambridge Philosophical Society.
Figures


References
-
- Aigaki, T. , Fleischmann, I. , Chen, P. S. & Kubli, E. (1991). Ectopic expression of sex peptide alters reproductive behavior of female D. melanogaster . Neuron 7, 557–563. - PubMed
-
- Alonzo, S. H. & Pizzari, T. (2010). Male fecundity stimulation: conflict and cooperation within and between the sexes: model analyses and coevolutionary dynamics. American Naturalist 175, 174–185. - PubMed
-
- Andersson, M. (1994). Sexual Selection. Princeton University Press, Princeton.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

