Proton Pump Inhibitor Therapy for Eosinophilic Esophagitis: History, Mechanisms, Efficacy, and Future Directions
- PMID: 35250281
- PMCID: PMC8892718
- DOI: 10.2147/JAA.S274524
Proton Pump Inhibitor Therapy for Eosinophilic Esophagitis: History, Mechanisms, Efficacy, and Future Directions
Abstract
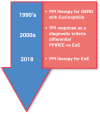
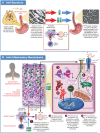
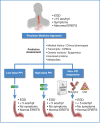
Over the past decade, the role of proton pump inhibitor (PPI) medication has evolved from a diagnostic tool for Eosinophilic Esophagitis (EoE), by excluding patients with PPI responsive esophageal eosinophilia (PPI-REE), to a therapy for EoE. This transition resulted from the Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the Appraisal of Guidelines for Research and Evaluation II (AGREE) Conference to support PPI therapy for EoE in children and adults. Additional recent advances have suggested a role for genetic variations that might impact response to PPI therapy for EoE. This review article will explore a brief background of EoE, the evolution of PPI therapy for EoE and its proposed mechanisms, efficacy and safety in children and adults, and considerations for future PPI precision medicine in patients with EoE.
Keywords: EoE; PPI; eosinophilic esophagitis; precision medicine; proton pump inhibitor medication.
© 2022 Franciosi et al.
Conflict of interest statement
JPF: No conflicts of interest to report. EBM: No conflicts of interest to report. ESD: Research funding: Adare/Ellodi, Allakos, Arena, AstraZeneca, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos/BMS, Regeneron, Shire/Takeda. Consultant: Abbott, AbbVie, Adare/Ellodi, Aimmune, Allakos, Amgen, Arena, AstraZeneca, Avir, Biorasi, Calypso, Celgene/Receptos/BMS, Celldex, Eli Lilly, EsoCap, GSK, Gossamer Bio, Landos, Morphic, Nutricia, Parexel/Calyx, Phathom, Regeneron, Revolo, Robarts/Alimentiv, Salix, Sanofi, Shire/Takeda. Educational grant: Allakos, Banner, Holoclara. CG-J: No conflicts of interest to report. SF-F: No conflicts of interest to report. RDV: No conflicts of interest to report. SKG: Consultant Abbott, Adare/Ellodi, Allakos, Celgene, Gossamer Bio, QOL, UpToDate, Medscape, Viaskin. Research support Shire; Allakos; Adare/Ellodi; NIH U54 grant to CEGIR.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

