Understanding Language Reorganization With Neuroimaging: How Language Adapts to Different Focal Lesions and Insights Into Clinical Applications
- PMID: 35250510
- PMCID: PMC8895248
- DOI: 10.3389/fnhum.2022.747215
Understanding Language Reorganization With Neuroimaging: How Language Adapts to Different Focal Lesions and Insights Into Clinical Applications
Abstract
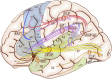
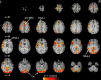
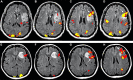
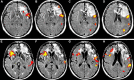
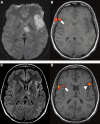
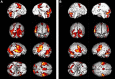
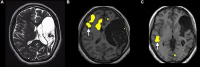
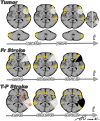
When the language-dominant hemisphere is damaged by a focal lesion, the brain may reorganize the language network through functional and structural changes known as adaptive plasticity. Adaptive plasticity is documented for triggers including ischemic, tumoral, and epileptic focal lesions, with effects in clinical practice. Many questions remain regarding language plasticity. Different lesions may induce different patterns of reorganization depending on pathologic features, location in the brain, and timing of onset. Neuroimaging provides insights into language plasticity due to its non-invasiveness, ability to image the whole brain, and large-scale implementation. This review provides an overview of language plasticity on MRI with insights for patient care. First, we describe the structural and functional language network as depicted by neuroimaging. Second, we explore language reorganization triggered by stroke, brain tumors, and epileptic lesions and analyze applications in clinical diagnosis and treatment planning. By comparing different focal lesions, we investigate determinants of language plasticity including lesion location and timing of onset, longitudinal evolution of reorganization, and the relationship between structural and functional changes.
Keywords: DTI—diffusion tensor imaging; epilepsy; fMRI; language; plasticity; reorganization; stroke; tumor.
Copyright © 2022 Pasquini, Di Napoli, Rossi-Espagnet, Visconti, Napolitano, Romano, Bozzao, Peck and Holodny.
Conflict of interest statement
AH is the Owner/President of fMRI Consultants, LLC, a purely educational entity. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Aerts A., Batens K., Santens P., van Mierlo P., Huysman E., Hartsuiker R., et al. (2015). Aphasia therapy early after stroke: behavioural and neurophysiological changes in the acute and post-acute phases. Aphasiology 29 845–871. 10.1080/02687038.2014.996520 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

