Functional Genomic Analysis of Amphetamine Sensitivity in Drosophila
- PMID: 35250674
- PMCID: PMC8894854
- DOI: 10.3389/fpsyt.2022.831597
Functional Genomic Analysis of Amphetamine Sensitivity in Drosophila
Abstract
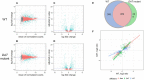
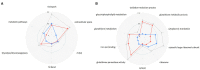
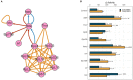
Abuse of psychostimulants, including amphetamines (AMPHs), is a major public health problem with profound psychiatric, medical, and psychosocial complications. The actions of these drugs at the dopamine transporter (DAT) play a critical role in their therapeutic efficacy as well as their liability for abuse and dependence. To date, however, the mechanisms that mediate these actions are not well-understood, and therapeutic interventions for AMPH abuse have been limited. Drug exposure can induce broad changes in gene expression that can contribute to neuroplasticity and effect long-lasting changes in neuronal function. Identifying genes and gene pathways perturbed by drug exposure is essential to our understanding of the molecular basis of drug addiction. In this study, we used Drosophila as a model to examine AMPH-induced transcriptional changes that are DAT-dependent, as those would be the most relevant to the stimulatory effects of the drug. Using this approach, we found genes involved in the control of mRNA translation to be significantly upregulated in response to AMPH in a DAT-dependent manner. To further prioritize genes for validation, we explored functional convergence between these genes and genes we identified in a genome-wide association study of AMPH sensitivity using the Drosophila Genetic Reference Panel. We validated a number of these genes by showing that they act specifically in dopamine neurons to mediate the behavioral effects of AMPH. Taken together, our data establish Drosophila as a powerful model that enables the integration of behavioral, genomic and transcriptomic data, followed by rapid gene validation, to investigate the molecular underpinnings of psychostimulant action.
Keywords: DGRP; Drosophila; S6K (70-kDa ribosomal protein S6 kinase); amphetamine; dopamine transporter; mammalian target of rapamycin (mTOR); psychostimulants; transcriptomic (RNA-Seq).
Copyright © 2022 Karam, Williams, Morozova, Yuan, Panarsky, Zhang, Hodgkinson, Goldman, Kalachikov and Javitch.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

