Biogenesis and Biological Functions of Extracellular Vesicles in Cellular and Organismal Communication With Microbes
- PMID: 35250933
- PMCID: PMC8895202
- DOI: 10.3389/fmicb.2022.817844
Biogenesis and Biological Functions of Extracellular Vesicles in Cellular and Organismal Communication With Microbes
Abstract
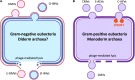
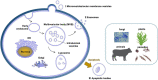
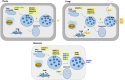
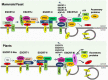
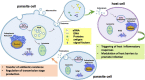
Extracellular vesicles (EVs) represent a prominent mechanism of transport and interaction between cells, especially microbes. Increasing evidence indicates that EVs play a key role in the physiological and pathological processes of pathogens and other symbionts. Recent research has focused on the specific functions of these vesicles during pathogen-host interactions, including trans-kingdom delivery of small RNAs, proteins and metabolites. Much current research on the function of EVs is focused on immunity and the interactions of microbes with human cells, while the roles of EVs during plant-microbe interactions have recently emerged in importance. In this review, we summarize recent research on the biogenesis of these vesicles and their functions in biology and pathology. Many key questions remain unclear, including the full structural and functional diversity of EVs, the roles of EVs in communication among microbes within microbiomes, how specific cargoes are targeted to EVs, whether EVs are targeted to specific destinations, and the full scope of EVs' transport of virulence effectors and of RNA and DNA molecules.
Keywords: biogenesis; cell to cell communication; extracellular vesicles (EVs); pathogen-plant interaction; pathology.
Copyright © 2022 Fang, Wang, Liu and Tyler.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Baltazar L. M., Zamith-Miranda D., Burnet M. C., Choi H., Nimrichter L., Nakayasu E. S., et al. (2018). Concentration-dependent protein loading of extracellular vesicles released by Histoplasma capsulatum after antibody treatment and its modulatory action upon macrophages. Sci. Rep. 8:8065. 10.1038/s41598-018-25665-5 - DOI - PMC - PubMed
-
- Bielaszewska M., Ruter C., Bauwens A., Greune L., Jarosch K. A., Steil D., et al. (2017). Host cell interactions of outer membrane vesicle-associated virulence factors of enterohemorrhagic Escherichia coli O157: intracellular delivery, trafficking and mechanisms of cell injury. PLoS Pathog. 13:e1006159. 10.1371/journal.ppat.1006159 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

