Consistent Biofilm Formation by Streptococcus pyogenes emm 1 Isolated From Patients With Necrotizing Soft Tissue Infections
- PMID: 35250938
- PMCID: PMC8895234
- DOI: 10.3389/fmicb.2022.822243
Consistent Biofilm Formation by Streptococcus pyogenes emm 1 Isolated From Patients With Necrotizing Soft Tissue Infections
Abstract
Objectives: Biofilm formation has been demonstrated in muscle and soft tissue samples from patients with necrotizing soft tissue infection (NSTI) caused by Streptococcus pyogenes, but the clinical importance of this observation is not clear. Although M-protein has been shown to be important for in vitro biofilm formation in S. pyogenes, the evidence for an association between emm type and biofilm forming capacity is conflicting. Here we characterize the biofilm forming capacity in a collection of S. pyogenes isolates causing NSTI, and relate this to emm type of the isolates and clinical characteristics of the patients.
Methods: Bacterial isolates and clinical data were obtained from NSTI patients enrolled in a multicenter prospective observational study. Biofilm forming capacity was determined using a microtiter plate assay.
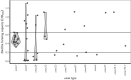
Results: Among 57 cases, the three most frequently encountered emm types were emm1 (n = 22), emm3 (n = 13), and emm28 (n = 7). The distribution of biofilm forming capacity in emm1 was qualitatively (narrow-ranged normal distribution) and quantitatively (21/22 isolates in the intermediate range) different from other emm types (wide ranged, multimodal distribution with 5/35 isolates in the same range as emm1). There were no significant associations between biofilm forming capacity and clinical characteristics of the patients.
Conclusions: The biofilm forming capacity of emm1 isolates was uniform and differed significantly from other emm types. The impact of biofilm formation in NSTI caused by S. pyogenes on clinical outcomes remains uncertain.
Keywords: M-protein; Streptococcus pyogenes; biofilms; emm1; necrotizing soft tissue infection (NSTI).
Copyright © 2022 Skutlaberg, Wiker, Mylvaganam, The INFECT Study Group, Norrby-Teglund and Skrede.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Baldassarri L., Creti R., Recchia S., Imperi M., Facinelli B., Giovanetti E., et al. (2006). Therapeutic failures of antibiotics used to treat macrolide-susceptible Streptococcus pyogenes infections may be due to biofilm formation. J. Clin. Microbiol. 44 2721–2727. 10.1128/JCM.00512-06 - DOI - PMC - PubMed
-
- Carroll R. K., Shelburne S. A., III, Olsen R. J., Suber B., Sahasrabhojane P., Kumaraswami M., et al. (2011). Naturally occurring single amino acid replacements in a regulatory protein alter streptococcal gene expression and virulence in mice. J. Clin. Invest. 121 1956–1968. 10.1172/JCI45169 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

