Physiological and Pathophysiological Roles of Metabolic Pathways for NET Formation and Other Neutrophil Functions
- PMID: 35251008
- PMCID: PMC8889909
- DOI: 10.3389/fimmu.2022.826515
Physiological and Pathophysiological Roles of Metabolic Pathways for NET Formation and Other Neutrophil Functions
Abstract
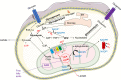
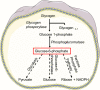
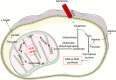
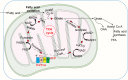
Neutrophils are the most numerous cells in the leukocyte population and essential for innate immunity. To limit their effector functions, neutrophils are able to modulate glycolysis and other cellular metabolic pathways. These metabolic pathways are essential not only for energy usage, but also for specialized effector actions, such as the production of reactive oxygen species (ROS), chemotaxis, phagocytosis, degranulation, and the formation of neutrophil extracellular traps (NETs). It has been demonstrated that activated viable neutrophils can produce NETs, which consists of a DNA scaffold able to bind granule proteins and microorganisms. The formation of NETs requires the availability of increased amounts of adenosine triphosphate (ATP) as it is an active cellular and therefore energy-dependent process. In this article, we discuss the glycolytic and other metabolic routes in association with neutrophil functions focusing on their role for building up NETs in the extracellular space. A better understanding of the requirements of metabolic pathways for neutrophil functions may lead to the discovery of molecular targets suitable to develop novel anti-infectious and/or anti-inflammatory drugs.
Keywords: glycolysis; metabolic switch; metabolism; neutrophil; neutrophil extracellular traps.
Copyright © 2022 Stojkov, Gigon, Peng, Lukowski, Ruth, Karaulov, Rizvanov, Barlev, Yousefi and Simon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Neutrophil Extracellular Traps Activate Proinflammatory Functions of Human Neutrophils.Front Immunol. 2021 Jun 8;12:636954. doi: 10.3389/fimmu.2021.636954. eCollection 2021. Front Immunol. 2021. PMID: 34168641 Free PMC article.
-
Metabolic Pathways Involved in Formation of Spontaneous and Lipopolysaccharide-Induced Neutrophil Extracellular Traps (NETs) Differ in Obesity and Systemic Inflammation.Int J Mol Sci. 2021 Jul 19;22(14):7718. doi: 10.3390/ijms22147718. Int J Mol Sci. 2021. PMID: 34299338 Free PMC article.
-
Metabolic requirements for neutrophil extracellular traps formation.Immunology. 2015 Jun;145(2):213-24. doi: 10.1111/imm.12437. Immunology. 2015. PMID: 25545227 Free PMC article.
-
Mechanisms of Neutrophil Extracellular Trap Formation and Regulation in Cancers.Int J Mol Sci. 2023 Jun 17;24(12):10265. doi: 10.3390/ijms241210265. Int J Mol Sci. 2023. PMID: 37373412 Free PMC article. Review.
-
Untangling "NETosis" from NETs.Eur J Immunol. 2019 Feb;49(2):221-227. doi: 10.1002/eji.201747053. Epub 2019 Jan 15. Eur J Immunol. 2019. PMID: 30629284 Review.
Cited by
-
NET Proteome in Established Type 1 Diabetes Is Enriched in Metabolic Proteins.Cells. 2023 May 5;12(9):1319. doi: 10.3390/cells12091319. Cells. 2023. PMID: 37174719 Free PMC article.
-
Syntaxin-4 and SNAP23 are involved in neutrophil degranulation, but not in the release of mitochondrial DNA during NET formation.Front Immunol. 2023 Oct 9;14:1272699. doi: 10.3389/fimmu.2023.1272699. eCollection 2023. Front Immunol. 2023. PMID: 37885878 Free PMC article.
-
Targeting Gαi2 in neutrophils protects from myocardial ischemia reperfusion injury.Basic Res Cardiol. 2024 Oct;119(5):717-732. doi: 10.1007/s00395-024-01057-x. Epub 2024 May 30. Basic Res Cardiol. 2024. PMID: 38811421 Free PMC article.
-
Tumor-neutrophil crosstalk promotes in vitro and in vivo glioblastoma progression.Front Immunol. 2023 May 24;14:1183465. doi: 10.3389/fimmu.2023.1183465. eCollection 2023. Front Immunol. 2023. PMID: 37292196 Free PMC article.
-
Oxidative Stress and Hyper-Inflammation as Major Drivers of Severe COVID-19 and Long COVID: Implications for the Benefit of High-Dose Intravenous Vitamin C.Front Pharmacol. 2022 Apr 29;13:899198. doi: 10.3389/fphar.2022.899198. eCollection 2022. Front Pharmacol. 2022. PMID: 35571085 Free PMC article. Review.

