Development of a Dendritic Cell/Tumor Cell Fusion Cell Membrane Nano-Vaccine for the Treatment of Ovarian Cancer
- PMID: 35251013
- PMCID: PMC8893350
- DOI: 10.3389/fimmu.2022.828263
Development of a Dendritic Cell/Tumor Cell Fusion Cell Membrane Nano-Vaccine for the Treatment of Ovarian Cancer
Abstract
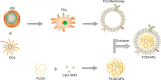
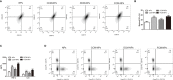
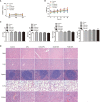
Ovarian cancer (OC) is a malignant tumor that seriously affects women's health. In recent years, immunotherapy has shown great potential in tumor treatment. As a major contributor of immunotherapy, dendritic cells (DCs) - based tumor vaccine has been demonstrated to have a positive effect in inducing immune responses in animal experiments. However, the effect of tumor vaccines in clinical trials is not ideal. Therefore, it is urgent to improve the existing tumor vaccines for tumor treatment. Here, we developed a fusion cell membrane (FCM) nano-vaccine FCM-NPs, which is prepared by fusing DCs and OC cells and coating the FCM on the poly (lactic-co-glycolic acid) (PLGA) nanoparticles (NPs) loaded with the immune adjuvant CpG-oligodeoxynucleotide (CpG-ODN). The fusion process promoted the maturation of DCs, thus up-regulating the expression of costimulatory molecule CD80/CD86 and accelerating lymph node homing of DCs. Furthermore, FCM-NPs has both the immunogenicity of tumor cells and the antigen presenting ability of DCs, it can stimulate naive T lymphocytes to produce a large number of tumor-specific cytotoxic CD8+ T lymphocytes. FCM-NPs exhibited strong immuno-activating effect both in vitro and in vivo. By establishing subcutaneous transplanted tumor model, patient-derived xenograft tumor model and abdominal metastatic tumor model, FCM-NPs was proved to have the effect of delaying the growth and inhibiting the metastasis of OC. FCM-NPs is expected to become a new tumor vaccine for the treatment of ovarian cancer.
Keywords: CpG-ODN; cytotoxic T lymphocytes; dendritic cell; fusion cell membrane; ovarian cancer.
Copyright © 2022 Zhang, Zhao, Huang, Li, Sheng, Song and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Alard E, Butnariu AB, Grillo M, Kirkham C, Zinovkin DA, Newnham L, et al. . Advances in Anti-Cancer Immunotherapy: Car-T Cell, Checkpoint Inhibitors, Dendritic Cell Vaccines, and Oncolytic Viruses, and Emerging Cellular and Molecular Targets. Cancers (Basel) (2020) 12(7):1826. doi: 10.3390/cancers12071826 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

