LPS Guides Distinct Patterns of Training and Tolerance in Mast Cells
- PMID: 35251027
- PMCID: PMC8891506
- DOI: 10.3389/fimmu.2022.835348
LPS Guides Distinct Patterns of Training and Tolerance in Mast Cells
Abstract
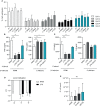
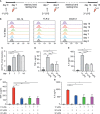
Mast cells (MCs) are tissue-resident, long lived innate immune cells with important effector and immunomodulatory functions. They are equipped with an eclectic variety of receptors that enable them to sense multiple stimuli and to generate specific responses according on the type, strength and duration of the stimulation. Several studies demonstrated that myeloid cells can retain immunological memory of their encounters - a process termed 'trained immunity' or 'innate immune memory'. As MCs are among the one of first cells to come into contact with the external environment, it is possible that such mechanisms of innate immune memory might help shaping their phenotype and effector functions; however, studies on this aspect of MC biology are still scarce. In this manuscript, we investigated the ability of MCs primed with different stimuli to respond to a second stimulation with the same or different ligands, and determined the molecular and epigenetic drivers of these responses. Our results showed that, while the stimulation with IgE and β-glucan failed to induce either tolerant or trained phenotypes, LPS conditioning was able to induce a profound and long-lasting remodeling of the signaling pathways involved in the response against LPS or fungal pathogens. On one side, LPS induced a strong state of unresponsiveness to secondary LPS stimulation due to the impairment of the PI3K-AKT signaling pathway, which resulted in the reduced activation of NF-κB and the decreased release of TNF-α and IL-6, compared to naïve MCs. On the other side, LPS primed MCs showed an increased release of TNF-α upon fungal infection with live Candida albicans, thus suggesting a dual role of LPS in inducing both tolerance and training phenotypes depending on the secondary challenge. Interestingly, the inhibition of HDAC during LPS stimulation partially restored the response of LPS-primed MCs to a secondary challenge with LPS, but failed to revert the increased cytokine production of these cells in response to C. albicans. These data indicate that MCs, as other innate immune cells, can develop innate immune memory, and that different stimulatory environments can shape and direct MC specific responses towards the dampening or the propagation of the local inflammatory response.
Keywords: Candida albicans; LPS; cytokines; endotoxin tolerance; mast cell; trained immunity.
Copyright © 2022 De Zuani, Dal Secco, Tonon, Arzese, Pucillo and Frossi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Ifrim CD, Quintin J, Joosten L, Jacobs C, Jansen T, Jacobs L, et al. . Mihai G Netea. Trained Immunity or Tolerance: Opposing Functional Programs Induced in Human Monocytes After Engagement of Various Pattern Recognition Receptors. Clin Vaccine Immunol (2014) 21(4):534–45. doi: 10.1128/CVI.00688-13 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

