Prophylaxis of cancer-associated venous thromboembolism with low-molecular-weight heparin-tinzaparin: Real world evidence
- PMID: 35251346
- PMCID: PMC8850961
- DOI: 10.3892/ol.2022.13235
Prophylaxis of cancer-associated venous thromboembolism with low-molecular-weight heparin-tinzaparin: Real world evidence
Abstract
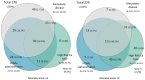
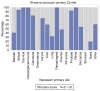
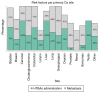
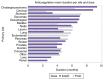
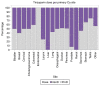
Thromboprophylaxis, as a preventive measure for cancer-associated thrombosis (CAT), may be beneficial for patients with active cancer and high-risk for thrombosis. The present post hoc analysis include a total of 407 patients enrolled in the Greek Management of Thrombosis study, who received thromboprophylaxis with tinzaparin. The objectives of the present analysis were: i) To obtain sufficient evidence for the administration of prophylaxis in patients with active cancer, irrespective of Khorana risk assessment model score; ii) to identify the selection criteria for both dose and duration of tinzaparin; and iii) to evaluate the efficacy and safety of tinzaparin administered for CAT prophylaxis. The main tumor types for the patients included in the present study were as follows: Lung (25.1%), pancreatic (14.3%), breast (9.1%), stomach (8.4%), colorectal (7.9%) and ovarian (7.6%). Furthermore, metastatic disease was observed in 69.5% of the patients. High thrombotic burden agents (HTBAs) were administered to 66.3% of the patients, and 17.4% received erythropoietin. A total of 43.7% of the patients exhibited a Khorana score <2. The results of the present study demonstrated that both the presence of metastatic disease and the use of HTBAs seemed to influence oncologists' decisions for the use of thromboprophylaxis in patients with active cancer, regardless of Khorana score. Tinzaparin, in dose expressed in the standard notation for heparins, i.e., anti-Xa factor international units (Anti-Xa IU), was administered at an intermediate dose (InterD; 8,000-12,000 Anti-Xa IU; once daily) to 52.4% of patients, while the remaining patients received a prophylactic dose (ProD; ≤4,500 Anti-Xa IU; once daily). The average duration of thromoprophylaxis was 5 months. Furthermore, a total of 14 (3.4%) thrombotic events and 6 (1.5%) minor bleeding events were recorded. A total of four thrombotic events were observed following an InterD treatment of tinzaparin, while 10 thrombotic events were observed following ProD treatment. The present study also demonstrated that an InterD of tinzaparin was administered more frequently to patients with a body mass index >30 kg/m2, a history of smoking and a history of metastatic disease, along with administration of erythropoietin. InterD tinzaparin treatment was found to be potentially more efficacious and without safety concerns. The present study is a registered clinical trial (ClinicalTrials.gov code, NCT03292107; registration date, September 25, 2017).
Keywords: active cancer; cancer associated thrombosis; low molecular weight heparins; prophylaxis; thrombosis; tinzaparin.
Copyright: © Christopoulou et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- National Institute for Health and Care Exellence, corp-author. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. https://www.nice.org.uk/guidance/ng158/resources/venous-thromboembolic-d.... [ November 10; 2021 ]; - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
