Tolerable upper intake level for dietary sugars
- PMID: 35251356
- PMCID: PMC8884083
- DOI: 10.2903/j.efsa.2022.7074
Tolerable upper intake level for dietary sugars
Abstract
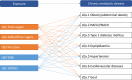
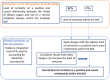
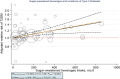
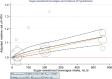
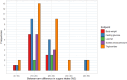
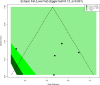
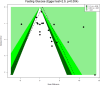
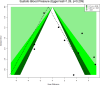
Following a request from five European Nordic countries, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) was tasked to provide scientific advice on a tolerable upper intake level (UL) or a safe level of intake for dietary (total/added/free) sugars based on available data on chronic metabolic diseases, pregnancy-related endpoints and dental caries. Specific sugar types (fructose) and sources of sugars were also addressed. The intake of dietary sugars is a well-established hazard in relation to dental caries in humans. Based on a systematic review of the literature, prospective cohort studies do not support a positive relationship between the intake of dietary sugars, in isocaloric exchange with other macronutrients, and any of the chronic metabolic diseases or pregnancy-related endpoints assessed. Based on randomised control trials on surrogate disease endpoints, there is evidence for a positive and causal relationship between the intake of added/free sugars and risk of some chronic metabolic diseases: The level of certainty is moderate for obesity and dyslipidaemia (> 50-75% probability), low for non-alcoholic fatty liver disease and type 2 diabetes (> 15-50% probability) and very low for hypertension (0-15% probability). Health effects of added vs. free sugars could not be compared. A level of sugars intake at which the risk of dental caries/chronic metabolic diseases is not increased could not be identified over the range of observed intakes, and thus, a UL or a safe level of intake could not be set. Based on available data and related uncertainties, the intake of added and free sugars should be as low as possible in the context of a nutritionally adequate diet. Decreasing the intake of added and free sugars would decrease the intake of total sugars to a similar extent. This opinion can assist EU Member States in setting national goals/recommendations.
Keywords: Tolerable upper intake level; added sugars; chronic metabolic diseases; dental caries; free sugars; pregnancy‐related endpoints; safe level of intake.
© 2022 Wiley‐VCH Verlag GmbH & Co. KgaA on behalf of the European Food Safety Authority.
Figures

















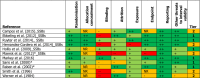

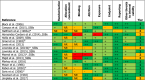



References
-
- Ahmadi‐Abhari S, Luben RN, Powell N, Bhaniani A, Chowdhury R, Wareham NJ, Forouhi NG and Khaw KT, 2014. Dietary intake of carbohydrates and risk of type 2 diabetes: the European Prospective Investigation into Cancer‐Norfolk study. British Journal of Nutrition, 111, 342–352. - PubMed
-
- Ahuja J and Perloff B, 2008. Quality control procedures for the USDA Food and Nutrient Database for Dietary Studies nutrient values. Journal of Food Composition and Analysis, 21.
LinkOut - more resources
Full Text Sources
