Design of therapeutic biomaterials to control inflammation
- PMID: 35251702
- PMCID: PMC8884103
- DOI: 10.1038/s41578-022-00426-z
Design of therapeutic biomaterials to control inflammation
Abstract
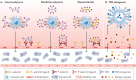
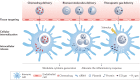
Inflammation plays an important role in the response to danger signals arising from damage to our body and in restoring homeostasis. Dysregulated inflammatory responses occur in many diseases, including cancer, sepsis and autoimmunity. The efficacy of anti-inflammatory drugs, developed for the treatment of dysregulated inflammation, can be potentiated using biomaterials, by improving the bioavailability of drugs and by reducing side effects. In this Review, we first outline key elements and stages of the inflammatory environment and then discuss the design of biomaterials for different anti-inflammatory therapeutic strategies. Biomaterials can be engineered to scavenge danger signals, such as reactive oxygen and nitrogen species and cell-free DNA, in the early stages of inflammation. Materials can also be designed to prevent adhesive interactions of leukocytes and endothelial cells that initiate inflammatory responses. Furthermore, nanoscale platforms can deliver anti-inflammatory agents to inflammation sites. We conclude by discussing the challenges and opportunities for biomaterial innovations in addressing inflammation.
Keywords: Biomedical materials.
© Springer Nature Limited 2022.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
