Tris(2-Pyridylmethylamine)V(O)2 Complexes as Counter Ions of Diprotonated Decavanadate Anion: Potential Antineoplastic Activity
- PMID: 35252118
- PMCID: PMC8888438
- DOI: 10.3389/fchem.2022.830511
Tris(2-Pyridylmethylamine)V(O)2 Complexes as Counter Ions of Diprotonated Decavanadate Anion: Potential Antineoplastic Activity
Abstract
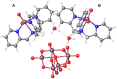
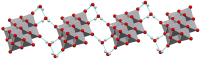
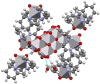
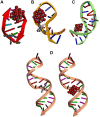
The synthesis and theoretical-experimental characterization of a novel diprotanated decavanadate is presented here due to our search for novel anticancer metallodrugs. Tris(2-pyridylmethyl)amine (TPMA), which is also known to have anticancer activity in osteosarcoma cell lines, was introduced as a possible cationic species that could act as a counterpart for the decavanadate anion. However, the isolated compound contains the previously reported vanadium (V) dioxido-tpma moieties, and the decavanadate anion appears to be diprotonated. The structural characterization of the compound was performed by infrared spectroscopy and single-crystal X-ray diffraction. In addition, DFT calculations were used to analyze the reactive sites involved in the donor-acceptor interactions from the molecular electrostatic potential maps. The level of theory mPW1PW91/6-31G(d)-LANL2DZ and ECP = LANL2DZ for the V atom was used. These insights about the compounds' main interactions were supported by analyzing the noncovalent interactions utilizing the AIM and Hirshfeld surfaces approach. Molecular docking studies with small RNA fragments were used to assess the hypothesis that decavanadate's anticancer activity could be attributed to its interaction with lncRNA molecules. Thus, a combination of three potentially beneficial components could be evaluated in various cancer cell lines.
Keywords: DFT calculations; TPMA; antineoplastic activity; decavanadate; molecular docking; vanadium (V) dioxido compounds.
Copyright © 2022 Corona-Motolinia, Martínez-Valencia, Noriega, Sánchez-Gaytán, Melendez, García-García, Choquesillo-Lazarte, Rodríguez-Diéguez, Castro and González-Vergara.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Synthesis, physicochemical and pharmacological characterizations of a tetra-[methylimidazolium] dihydrogen decavanadate, inhibiting the IGR39 human melanoma cells development.J Inorg Biochem. 2024 Nov;260:112672. doi: 10.1016/j.jinorgbio.2024.112672. Epub 2024 Jul 25. J Inorg Biochem. 2024. PMID: 39079338
-
An EXAFS Approach to the Study of Polyoxometalate-Protein Interactions: The Case of Decavanadate-Actin.Inorg Chem. 2017 Sep 18;56(18):10893-10903. doi: 10.1021/acs.inorgchem.7b01018. Epub 2017 Aug 31. Inorg Chem. 2017. PMID: 28858484
-
Decavanadate Salts of Cytosine and Metformin: A Combined Experimental-Theoretical Study of Potential Metallodrugs Against Diabetes and Cancer.Front Chem. 2018 Oct 2;6:402. doi: 10.3389/fchem.2018.00402. eCollection 2018. Front Chem. 2018. PMID: 30333969 Free PMC article.
-
Decavanadate (V10 O28 6-) and oxovanadates: oxometalates with many biological activities.J Inorg Biochem. 2009 Apr;103(4):536-46. doi: 10.1016/j.jinorgbio.2008.11.010. Epub 2008 Nov 28. J Inorg Biochem. 2009. PMID: 19110314 Review.
-
Recent advances into vanadyl, vanadate and decavanadate interactions with actin.Metallomics. 2012 Jan;4(1):16-22. doi: 10.1039/c1mt00124h. Epub 2011 Oct 19. Metallomics. 2012. PMID: 22012168 Review.
Cited by
-
In Vitro, Oral Acute, and Repeated 28-Day Oral Dose Toxicity of a Mixed-Valence Polyoxovanadate Cluster.Pharmaceuticals (Basel). 2023 Aug 30;16(9):1232. doi: 10.3390/ph16091232. Pharmaceuticals (Basel). 2023. PMID: 37765040 Free PMC article.
References
-
- Adamo C., Barone V. (1998). Exchange Functionals with Improved Long-Range Behavior and Adiabatic Connection Methods without Adjustable Parameters: The mPW and mPW1PW Models. J. Chem. Phys. 108, 664–675. 10.1063/1.475428 - DOI
-
- Amante C., De Sousa-Coelho A. L., Aureliano M., Aureliano M. (2021). Vanadium and Melanoma: A Systematic Review. Metals 11 (5), 828. 10.3390/met11050828 - DOI
LinkOut - more resources
Full Text Sources

