Role of ATP-Small Heat Shock Protein Interaction in Human Diseases
- PMID: 35252358
- PMCID: PMC8890618
- DOI: 10.3389/fmolb.2022.844826
Role of ATP-Small Heat Shock Protein Interaction in Human Diseases
Abstract
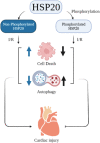
Adenosine triphosphate (ATP) is an important fuel of life for humans and Mycobacterium species. Its potential role in modulating cellular functions and implications in systemic, pulmonary, and ocular diseases is well studied. Plasma ATP has been used as a diagnostic and prognostic biomarker owing to its close association with disease's progression. Several stresses induce altered ATP generation, causing disorders and illnesses. Small heat shock proteins (sHSPs) are dynamic oligomers that are dominantly β-sheet in nature. Some important functions that they exhibit include preventing protein aggregation, enabling protein refolding, conferring thermotolerance to cells, and exhibiting anti-apoptotic functions. Expression and functions of sHSPs in humans are closely associated with several diseases like cataracts, cardiovascular diseases, renal diseases, cancer, etc. Additionally, there are some mycobacterial sHSPs like Mycobacterium leprae HSP18 and Mycobacterium tuberculosis HSP16.3, whose molecular chaperone functions are implicated in the growth and survival of pathogens in host species. As both ATP and sHSPs, remain closely associated with several human diseases and survival of bacterial pathogens in the host, therefore substantial research has been conducted to elucidate ATP-sHSP interaction. In this mini review, the impact of ATP on the structure and function of human and mycobacterial sHSPs is discussed. Additionally, how such interactions can influence the onset of several human diseases is also discussed.
Keywords: ATP; cardiovascular diseases; cataract; leprosy; sHSPs; tuberculosis.
Copyright © 2022 Nandi, Panda, Chakraborty, Rathee, Roy, Barik, Mohapatra and Biswas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Bredemeier M., Lopes L. M., Eisenreich M. A., Hickmann S., Bongiorno G. K., d'Avila R., et al. (2018). Xanthine Oxidase Inhibitors for Prevention of Cardiovascular Events: a Systematic Review and Meta-Analysis of Randomized Controlled Trials. BMC Cardiovasc. Disord. 18, 1824. 10.1186/s12872-018-0757-9 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials