A histological study of atherosclerotic characteristics in age-related macular degeneration
- PMID: 35252605
- PMCID: PMC8891972
- DOI: 10.1016/j.heliyon.2022.e08973
A histological study of atherosclerotic characteristics in age-related macular degeneration
Abstract
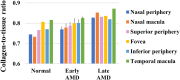
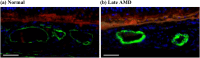
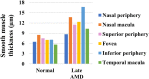
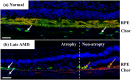
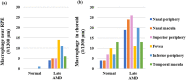
This study investigated the pathogenesis of age-related macular degeneration (AMD) using histological methods that are commonly used for atherosclerotic vascular disease (ASVD). 1 normal, 3 early dry AMD, and 1 late dry AMD eyes were obtained from the Lions Eye Bank of Oregon and systematically dissected. They were stained with hematoxylin and eosin, Oil red O, Masson, Elastica van Gieson, Alizarin red, and Prussian blue. Additionally, the normal and late dry AMD eyes were immunostained for a-smooth muscle actin, CD45, and CD68 with Nile red and DAPI. Correlations were found between severity of AMD and lipid accumulation in the deep sclera (+), numbers of drusen between the Bruch's membrane and retinal pigment epithelium (RPE) (+), amount of collagen in the deep sclera (+), and amount of elastin in the deep sclera (-) (P < 0.1). Geographic atrophy, RPE detachment, and abnormal capillary shape and distribution in the choriocapillaris were observed in the fovea of late AMD. There were no stenosis, plaque, hemorrhage, and calcification. Additionally, late AMD tended to have higher smooth muscle thicknesses of the choroidal vascular walls, lower numbers of T lymphocytes in the choroid, and higher numbers of macrophages near the RPE and in the choroid relative to normal (P < 0.1). Macrophages-derived foam cells were detected near the Bruch's membrane in late AMD. Therefore, the present study showed many histological characteristics of ASVD in AMD, which suggests an association between them; however, there were also some histological characteristics of ASVD that were not found in AMD, which indicates that there exist pathogenic differences between them. The results generally support the vascular model of AMD, but some details still need clarification.
Keywords: Atherosclerosis; Atherosclerotic vascular disease; Blood vessel; Histology; Staining.
© 2022 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
Histologic and morphometric analysis of the choroid, Bruch's membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes.Surv Ophthalmol. 1999 Oct;44 Suppl 1:S10-32. doi: 10.1016/s0039-6257(99)00086-7. Surv Ophthalmol. 1999. PMID: 10548114
-
Bruch's Membrane and the Choroid in Age-Related Macular Degeneration.Adv Exp Med Biol. 2021;1256:89-119. doi: 10.1007/978-3-030-66014-7_4. Adv Exp Med Biol. 2021. PMID: 33847999
-
[Age-related Macular Degeneration in the Japanese].Nippon Ganka Gakkai Zasshi. 2016 Mar;120(3):163-88; discussion 189. Nippon Ganka Gakkai Zasshi. 2016. PMID: 27164756 Japanese.
-
Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex.Mol Aspects Med. 2012 Aug;33(4):295-317. doi: 10.1016/j.mam.2012.04.005. Epub 2012 Apr 21. Mol Aspects Med. 2012. PMID: 22542780 Free PMC article. Review.
-
Changes in choriocapillaris and retinal pigment epithelium in age-related macular degeneration.Mol Vis. 1999 Nov 3;5:35. Mol Vis. 1999. PMID: 10562659 Review.
Cited by
-
Targeting the Complement Cascade for Treatment of Dry Age-Related Macular Degeneration.Biomedicines. 2022 Aug 4;10(8):1884. doi: 10.3390/biomedicines10081884. Biomedicines. 2022. PMID: 36009430 Free PMC article. Review.
-
Necroptosis and Neuroinflammation in Retinal Degeneration.Front Neurosci. 2022 Jun 29;16:911430. doi: 10.3389/fnins.2022.911430. eCollection 2022. Front Neurosci. 2022. PMID: 35844208 Free PMC article. Review.
-
Oxidative Stress and Lipid Accumulation Augments Cell Death in LDLR-Deficient RPE Cells and Ldlr-/- Mice.Cells. 2022 Dec 22;12(1):43. doi: 10.3390/cells12010043. Cells. 2022. PMID: 36611838 Free PMC article.
-
Assessing the role of T cells in response to retinal injury to uncover new therapeutic targets for the treatment of retinal degeneration.J Neuroinflammation. 2023 Sep 9;20(1):206. doi: 10.1186/s12974-023-02867-x. J Neuroinflammation. 2023. PMID: 37689689 Free PMC article.
-
Macrophages coordinate immune response to laser-induced injury via extracellular traps.J Neuroinflammation. 2024 Mar 18;21(1):68. doi: 10.1186/s12974-024-03064-0. J Neuroinflammation. 2024. PMID: 38500151 Free PMC article.
References
-
- Jonas J.B., Bourne R.R.A., White R.A., et al. Visual impairment and blindness due to macular diseases globally: a systematic review and meta-analysis. Am. J. Ophthalmol. 2014;158(4):808–815. - PubMed
-
- Jonas J.B., Cheung C.M.G., Panda-Jonas S. Updates on the epidemiology of age-related macular degeneration. Asia Pac. J. Ophthalmol. (Phila) 2017;6(6):493–497. - PubMed
-
- Wong W.L., Su X., Li X., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Global Health. 2014;2(2):e106–116. - PubMed
-
- Possek E. Ueber senile Maculaveränderung bei Arteriosklerose. Ophthalmologica. 1905;13(1):771–779.
-
- Vingerling J.R., Dielemans I., Bots M.L., et al. Age-related macular degeneration is associated with atherosclerosis. The Rotterdam Study. Am. J. Epidemiol. 1995;142(4):404–409. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

