Sebocytes contribute to melasma onset
- PMID: 35252805
- PMCID: PMC8891974
- DOI: 10.1016/j.isci.2022.103871
Sebocytes contribute to melasma onset
Abstract
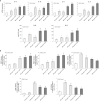
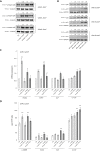
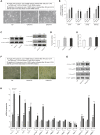
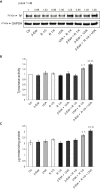
Melasma is a hyperpigmentary disorder with photoaging features, whose manifestations appear on specific face areas, rich in sebaceous glands (SGs). To explore the SGs possible contribution to the onset, the expression of pro-melanogenic and inflammatory factors from the SZ95 SG cell line exposed to single or repetitive ultraviolet (UVA) radiation was evaluated. UVA up-modulated the long-lasting production of α-MSH, EDN1, b-FGF, SCF, inflammatory cytokines and mediators. Irradiated SZ95 sebocyte conditioned media increased pigmentation in melanocytes and the expression of senescence markers, pro-inflammatory cytokines, and growth factors regulating melanogenesis in fibroblasts cultures. Cocultures experiments with skin explants confirmed the role of sebocytes on melanogenesis promotion. The analysis on sebum collected from melasma patients demonstrated that in vivo sebocytes from lesional areas express the UVA-activated pathways markers observed in vitro. Our results indicate sebocytes as one of the actors in melasma pathogenesis, inducing prolonged skin cell stimulation, contributing to localized dermal aging and hyperpigmentation.
Keywords: Biochemistry; Biological sciences; Molecular biology.
© 2022.
Conflict of interest statement
The authors declare no competing interests
Figures




References
-
- Abdel-Naser M.B., Seltmann H., Zouboulis C.C. SZ95 sebocytes induce epidermal melanocyte dendricity and proliferation in vitro. Exp. Dermatol. 2012;21:393–395. - PubMed
-
- Bak H., Lee H.J., Chang S.E., Choi J.H., Kim M.N., Kim B.J. Increased expression of nerve growth factor receptor and neural endopeptidase in the lesional skin of melasma. Dermatol. Surg. 2009;35:1244–1250. - PubMed
-
- Barker N. The canonical Wnt/beta-catenin signalling pathway. Methods Mol. Biol. 2008;468:5–15. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

