Antroquinonol administration in animal preclinical studies for Alzheimer's disease (AD): A new avenue for modifying progression of AD pathophysiology
- PMID: 35252893
- PMCID: PMC8892093
- DOI: 10.1016/j.bbih.2022.100435
Antroquinonol administration in animal preclinical studies for Alzheimer's disease (AD): A new avenue for modifying progression of AD pathophysiology
Abstract
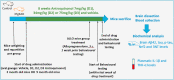
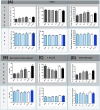
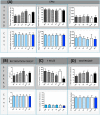
Despite the rise of Alzheimer's disease (AD) in an ageing population, no cure is currently available for this disorder. This study assessed the role of a natural compound, Antroquinonol, in modifying the progression of AD when administered at the start and/or before appearance of symptoms and when the disease was well established, in a transgenic animal model. Antroquinonol was administered daily for 8 weeks, in 11 week (early stage) and 9 month (late stage) male transgenic mice (3 times Transgenic mice PS1M146V, APPSwe, and tauP301L, 3 Tg XAD) and their respective aged controls. Behavioural testing (including Elevated Plus Maze Watermaze, Recognition object testing and Y maze) was performed at the end of the drug administration. In addition AD biomarkers (Amyloid beta 42 (Aβ42), tau and phospho-tau levels), oxidative stress and inflammatory markers, were assessed in tested mice brains after their sacrifice at the end of the treatment. When administered before the start of symptoms at 11 weeks, Antroquinonol treatment at 34 mg/kg (D2) and more consistently at 75 mg/kg (D3), had a significant effect on reducing systemic inflammatory markers (Interleukin 1, IL-1β and TNF-α) and AD biomarker (Amyloid Beta 42, Aβ42 and tau) levels in the brain. The reduction of behavioural impairment reported for 3TgXAD mice was observed significantly for the D3 drug dose only and for all behavioural tests, when administered at 11 weeks. Similarly, beneficial effects of Antroquinonol (at higher dose D3) were noted in the transgenic mice in terms of AD biomarkers (tau and phosphorylated-tau), systemic inflammatory (IL-1β), brain anti-inflammatory (Nrf2) and oxidative (3-Nitrotyrosine, 3NT) markers. Improvement of memory impairment was also reported when Antroquinonol (D3) was administered at late stage (9 months). Since Antroquinonol has been used without adverse effects in previous successful clinical trials, this drug may offer a new avenue of treatment to modify AD development and progression.
Keywords: AD biomarkers; AICD, APP Intracellular Cytoplasmic/C-terminal Domain; AMPK, 5′ adenosine monophosphate-activated protein kinase; APP, Amyloid precursor protein; APPβ, secreted amino-terminals APPβ fragment; Alzheimer's disease (AD); Animal model of AD; Antroquinonol; Aβ, amyloid-β peptides; Behavioural testing; CTFα, carboxyterminal fragment-α; CTFβ, carboxyterminal fragment-β; GSK3β, Glycogen synthase kinase 3 beta; IFNγ, Interferon gamma; IL-1, Interleukin 1; IL-6, Interleukin 6; Inflammatory markers; MAPK, Mitogen activated protein kinase; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; NMDA Rc, methyl-D-aspartate receptors receptor; ROS, reactive oxygen species; TNF-α, Tumor Necrosis factor alpha; Transgenic mice; cdck5, cyclin dependant kinase 5; sAPPα, secreted amino-terminals APPα fragment.
© 2022 Published by Elsevier Inc.
Figures


Similar articles
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
-
Antroquinonol Lowers Brain Amyloid-β Levels and Improves Spatial Learning and Memory in a Transgenic Mouse Model of Alzheimer's Disease.Sci Rep. 2015 Oct 15;5:15067. doi: 10.1038/srep15067. Sci Rep. 2015. PMID: 26469245 Free PMC article.
-
PTI-125 Reduces Biomarkers of Alzheimer's Disease in Patients.J Prev Alzheimers Dis. 2020;7(4):256-264. doi: 10.14283/jpad.2020.6. J Prev Alzheimers Dis. 2020. PMID: 32920628 Clinical Trial.
-
Hydrogen sulfide slows down progression of experimental Alzheimer's disease by targeting multiple pathophysiological mechanisms.Neurobiol Learn Mem. 2013 Sep;104:82-91. doi: 10.1016/j.nlm.2013.05.006. Epub 2013 May 31. Neurobiol Learn Mem. 2013. PMID: 23726868
-
Decoding Alzheimer's disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies.Prog Neurobiol. 2013 Sep;108:21-43. doi: 10.1016/j.pneurobio.2013.06.004. Epub 2013 Jul 11. Prog Neurobiol. 2013. PMID: 23850509 Review.
Cited by
-
NRF2 Deficiency Promotes Ferroptosis of Astrocytes Mediated by Oxidative Stress in Alzheimer's Disease.Mol Neurobiol. 2024 Oct;61(10):7517-7533. doi: 10.1007/s12035-024-04023-9. Epub 2024 Feb 24. Mol Neurobiol. 2024. PMID: 38401046
-
Fungal Bioactive Compounds as Emerging Therapeutic Options for Age-Related Neurodegenerative Disorders.Int J Mol Sci. 2025 May 16;26(10):4800. doi: 10.3390/ijms26104800. Int J Mol Sci. 2025. PMID: 40429941 Free PMC article. Review.
-
Current and further outlook on the protective potential of Antrodia camphorata against neurological disorders.Front Pharmacol. 2024 Apr 17;15:1372110. doi: 10.3389/fphar.2024.1372110. eCollection 2024. Front Pharmacol. 2024. PMID: 38694913 Free PMC article. Review.
References
-
- Abe J., Okuda M., Huang Q., Yoshizumi M., Berk B.C. Reactive oxygen species activate p90 ribosomal S6 kinase via Fyn and Ras. J. Biol. Chem. 2000;275(3):1739–1748. - PubMed
-
- Anand A., Patience A.A., Sharma N., Khurana N. The present and future of pharmacotherapy of Alzheimer's disease: a comprehensive review. Eur. J. Pharmacol. 2017;815:364–375. - PubMed
-
- Angamuthu V., Shanmugavadivu M., Nagarajan G., Velmurugan B.K. Pharmacological activities of antroquinonol- Mini review. Chem. Biol. Interact. 2019;297:8–15. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

