DEVELOPMENT OF A PORTABLE BLOOD POTASSIUM MONITORING DEVICE FOR DIALYSIS PATIENTS
- PMID: 35253014
- PMCID: PMC8895231
- DOI: 10.1115/dmd2020-9066
DEVELOPMENT OF A PORTABLE BLOOD POTASSIUM MONITORING DEVICE FOR DIALYSIS PATIENTS
Abstract
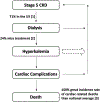
Approximately 500,000 dialysis patients in America are at a high risk of hyperkalemia, a condition where blood potassium becomes elevated above normal levels. Hyperkalemia is extremely dangerous, as it can result in severe cardiac complications if untreated. Hyperkalemia may be silent or present vague symptoms until those complications develop, at which point patients require emergency medical care. However, if patients have the ability to measure their potassium levels at home, they could detect hyperkalemia before it reaches a dangerous stage, and seek preventative medical care to avoid severe complications. Therefore, we have designed a novel device allowing patients to measure their blood potassium levels at home. The workflow of our solution is as follows: (1) patients obtain a blood sample from a finger prick, (2) potassium concentration is measured with an ion specific electrode (ISE), and (3) the device displays their potassium levels and a recommended course of action based on their hyperkalemic risk. We validate our solution with three major tests. First, our portable ISE technology must accurately measure potassium concentration in blood samples. Second, appropriate lancet parameters (gauge and depth) to minimize hemolysis in capillary blood samples must be found to minimize falsely elevated readings. Third, device portability and ease of use must be evaluated using patient input, as these factors will affect patient compliance. We have validated the use of portable ISE technology to feasibly measure potassium, and we continue to collect data for our second and third tests.
Figures

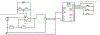
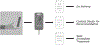
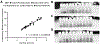
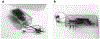

Similar articles
-
[Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Feb 12;47(2):101-119. doi: 10.3760/cma.j.cn112147-20231019-00247. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38309959 Chinese.
-
Prevalence and Prognosis of Hyperkalemia in Patients with Acute Myocardial Infarction.Am J Med. 2016 Aug;129(8):858-65. doi: 10.1016/j.amjmed.2016.03.008. Epub 2016 Apr 7. Am J Med. 2016. PMID: 27060233 Free PMC article.
-
Evaluation of 2 portable ion-selective electrode meters for determining whole blood, plasma, urine, milk, and abomasal fluid potassium concentrations in dairy cattle.J Dairy Sci. 2016 Sep;99(9):7330-7343. doi: 10.3168/jds.2015-10821. Epub 2016 Jul 7. J Dairy Sci. 2016. PMID: 27394952
-
POCT errors can lead to false potassium results.Adv Lab Med. 2021 Dec 30;3(2):142-152. doi: 10.1515/almed-2021-0079. eCollection 2022 Jun. Adv Lab Med. 2021. PMID: 37361872 Free PMC article. Review.
-
Therapeutic approach to hyperkalemia.Nephron. 2002;92 Suppl 1:33-40. doi: 10.1159/000065375. Nephron. 2002. PMID: 12401936 Review.
References
-
- Genovesi S, Valsecchi MG, Rossi E, Pogliani D, Acquistapace I, De Cristofaro V, Stella A, and Vincenti A, 2009, “Sudden death and associated factors in a historical cohort of chronic haemodialysis patients,” Nephrol Dial Transplant 24(8), pp. 2529–2536. - PubMed
-
- Brunelli SM, Du Mond C, Oestreicher N, Rakov V, and Spiegel DM, 2017, “Serum potassium and short-term clinical outcomes among hemodialysis patients: Impact of the long interdialytic interval,” Am J Kidney Dis, 70(1), pp. 21–29. - PubMed
-
- National Institutes of Health, 2017, “USRDS annual data report: Epidemiology of kidney disease in the United States,” from https://www.usrds.org/2016/view/Default.aspx.
-
- Vespa J, Armstrong DM, and Medina L, 2018, “Demographic Turning Points for the United States: Population Projections for 2020 to 2060 Population Estimates and Projections Current Population Reports,” from www.census.gov/programs-surveys/popproj.
Grants and funding
LinkOut - more resources
Full Text Sources
