Effects of Magnesium Citrate, Magnesium Oxide, and Magnesium Sulfate Supplementation on Arterial Stiffness: A Randomized, Double-Blind, Placebo-Controlled Intervention Trial
- PMID: 35253448
- PMCID: PMC9075273
- DOI: 10.1161/JAHA.121.021783
Effects of Magnesium Citrate, Magnesium Oxide, and Magnesium Sulfate Supplementation on Arterial Stiffness: A Randomized, Double-Blind, Placebo-Controlled Intervention Trial
Abstract
Background Magnesium supplements may have beneficial effects on arterial stiffness. Yet, to our knowledge, no head-to-head comparison between various magnesium formulations in terms of effects on arterial stiffness has been performed. We assessed the effects of magnesium citrate supplementation on arterial stiffness and blood pressure and explored whether other formulations of magnesium have similar effects. Methods and Results In this randomized trial, subjects who were overweight and slightly obese received either magnesium citrate, magnesium oxide, magnesium sulfate, or placebo for 24 weeks. The total daily dose of magnesium was 450 mg/d. The primary outcome was carotid-to-femoral pulse wave velocity, which is the gold standard method for measuring arterial stiffness. Secondary outcomes included blood pressure and plasma and urine magnesium. Overall, 164 participants (mean±SD age, 63.2±6.8 years; 104 [63.4%] women) were included. In the intention-to-treat analysis, neither magnesium citrate nor the other formulations had an effect on carotid-to-femoral pulse wave velocity or blood pressure at 24 weeks compared with placebo. Magnesium citrate increased plasma (+0.04 mmol/L; 95% CI, +0.02 to +0.06 mmol/L) and urine magnesium (+3.12 mmol/24 h; 95% CI, +2.23 to +4.01 mmol/24 h) compared with placebo. Effects on plasma magnesium were similar among the magnesium supplementation groups, but magnesium citrate led to a more pronounced increase in 24-hour urinary magnesium excretion than magnesium oxide or magnesium sulfate. One serious adverse event was reported, which was considered unrelated to the study treatment. Conclusions Oral magnesium citrate supplementation for 24 weeks did not significantly change arterial stiffness or blood pressure. Magnesium oxide and magnesium sulfate had similar nonsignificant effects. Registration URL: https://www.clinicaltrials.gov; Unique identifier: NCT03632590.
Keywords: arterial stiffness; blood pressure; intervention study; magnesium; pulse wave velocity; supplementation.
Figures
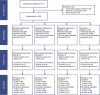
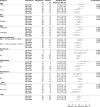
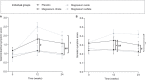
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

