Diverse functions for acyltransferase-3 proteins in the modification of bacterial cell surfaces
- PMID: 35253642
- PMCID: PMC9558356
- DOI: 10.1099/mic.0.001146
Diverse functions for acyltransferase-3 proteins in the modification of bacterial cell surfaces
Abstract
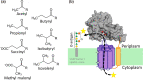
The acylation of sugars, most commonly via acetylation, is a widely used mechanism in bacteria that uses a simple chemical modification to confer useful traits. For structures like lipopolysaccharide, capsule and peptidoglycan, that function outside of the cytoplasm, their acylation during export or post-synthesis requires transport of an activated acyl group across the membrane. In bacteria this function is most commonly linked to a family of integral membrane proteins - acyltransferase-3 (AT3). Numerous studies examining production of diverse extracytoplasmic sugar-containing structures have identified roles for these proteins in O-acylation. Many of the phenotypes conferred by the action of AT3 proteins influence host colonisation and environmental survival, as well as controlling the properties of biotechnologically important polysaccharides and the modification of antibiotics and antitumour drugs by Actinobacteria. Herein we present the first systematic review, to our knowledge, of the functions of bacterial AT3 proteins, revealing an important protein family involved in a plethora of systems of importance to bacterial function that is still relatively poorly understood at the mechanistic level. By defining and comparing this set of functions we draw out common themes in the structure and mechanism of this fascinating family of membrane-bound enzymes, which, due to their role in host colonisation in many pathogens, could offer novel targets for the development of antimicrobials.
Keywords: O-acetylation; O-succinylation; acetyl-CoA; antibiotics; capsule; exopolysaccharide; lipopolysaccharide; membrane-bound acyltransferase; mycolic acid; nodulation.
Conflict of interest statement
All authors state that they have no conflicts of interest in preparing and submitting this manuscript. Prof. Thomas is the Editor in Chief of the journal and Dr. van der Woude is an Editor, although this should not influence how the manuscript is handled during its submission.
Figures

Similar articles
-
A novel fold for acyltransferase-3 (AT3) proteins provides a framework for transmembrane acyl-group transfer.Elife. 2023 Jan 11;12:e81547. doi: 10.7554/eLife.81547. Elife. 2023. PMID: 36630168 Free PMC article.
-
Acetylation of Surface Carbohydrates in Bacterial Pathogens Requires Coordinated Action of a Two-Domain Membrane-Bound Acyltransferase.mBio. 2020 Aug 25;11(4):e01364-20. doi: 10.1128/mBio.01364-20. mBio. 2020. PMID: 32843546 Free PMC article.
-
Mechanism of Staphylococcus aureus peptidoglycan O-acetyltransferase A as an O-acyltransferase.Proc Natl Acad Sci U S A. 2021 Sep 7;118(36):e2103602118. doi: 10.1073/pnas.2103602118. Proc Natl Acad Sci U S A. 2021. PMID: 34480000 Free PMC article.
-
Acyltransferases that Modify Cell Surface Polymers Across the Membrane.Biochemistry. 2025 Apr 15;64(8):1728-1749. doi: 10.1021/acs.biochem.4c00731. Epub 2025 Apr 2. Biochemistry. 2025. PMID: 40171682 Review.
-
Regulatory effects of post-translational modifications on zDHHC S-acyltransferases.J Biol Chem. 2020 Oct 23;295(43):14640-14652. doi: 10.1074/jbc.REV120.014717. Epub 2020 Aug 17. J Biol Chem. 2020. PMID: 32817054 Free PMC article. Review.
Cited by
-
Two novel genes identified by large-scale transcriptomic analysis are essential for biofilm and rugose colony development of Vibrio vulnificus.PLoS Pathog. 2023 Jan 19;19(1):e1011064. doi: 10.1371/journal.ppat.1011064. eCollection 2023 Jan. PLoS Pathog. 2023. PMID: 36656902 Free PMC article.
-
Inferior Caballeronia symbiont lacks conserved symbiosis genes.Microb Genom. 2024 Dec;10(12):001333. doi: 10.1099/mgen.0.001333. Microb Genom. 2024. PMID: 39680049 Free PMC article.
-
A novel fold for acyltransferase-3 (AT3) proteins provides a framework for transmembrane acyl-group transfer.Elife. 2023 Jan 11;12:e81547. doi: 10.7554/eLife.81547. Elife. 2023. PMID: 36630168 Free PMC article.
-
Involvement of a putative acyltransferase gene in sporangium formation in Actinoplanes missouriensis.Microbiol Spectr. 2024 May 2;12(5):e0401023. doi: 10.1128/spectrum.04010-23. Epub 2024 Mar 19. Microbiol Spectr. 2024. PMID: 38501822 Free PMC article.
-
Linking methanotroph phenotypes to genotypes using a simple spatially resolved model ecosystem.ISME J. 2024 Jan 8;18(1):wrae060. doi: 10.1093/ismejo/wrae060. ISME J. 2024. PMID: 38622932 Free PMC article.
References
-
- Shaw WV. The enzymatic acetylation of chloramphenicol by extracts of R factor-resistant Escherichia coli . J Biol Chem. 1967;242:687–693. - PubMed
-
- Slauch JM, Lee AA, Mahan MJ, Mekalanos JJ. Molecular characterization of the oafA locus responsible for acetylation of Salmonella typhimurium O-antigen: oafA is a member of a family of integral membrane trans-acylases. J Bacteriol. 1996;178:5904–5909. doi: 10.1128/jb.178.20.5904-5909.1996. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

