Metabolic dynamics instruct CD8+ T-cell differentiation and functions
- PMID: 35253907
- PMCID: PMC9314626
- DOI: 10.1002/eji.202149486
Metabolic dynamics instruct CD8+ T-cell differentiation and functions
Abstract
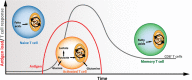
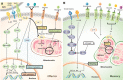
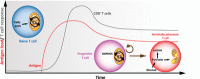
Cytotoxic CD8+ T cells are a key element of the adaptative immune system to protect the organism against infections and malignant cells. During their activation and response, T cells undergo different metabolic pathways to support their energetic needs according to their localization and function. However, it has also been recently appreciated that this metabolic reprogramming also directly supports T-cell lineage differentiation. Accordingly, metabolic deficiencies and prolonged stress exposure can impact T-cell differentiation and skew them into an exhausted state. Here, we review how metabolism defines CD8+ T-cell differentiation and function. Moreover, we cover the principal metabolic dysregulation that promotes the exhausted phenotype under tumor or chronic virus conditions. Finally, we summarize recent strategies to reprogram impaired metabolic pathways to promote CD8+ T-cell effector function and survival.
Keywords: T-cell differentiation; T-cell exhaustion; T cells; infection; metabolism.
© 2022 The Authors. European Journal of Immunology published by Wiley-VCH GmbH.
Conflict of interest statement
P.‐C.H. serves as scientific advisors for Elixiron Immunotherapeutics and Novartis. The rest of the authors confirm no commercial or financial conflict of interest.
Figures

References
-
- Almeida, L. , Lochner, M. , Berod, L. and Sparwasser, T. , Metabolic pathways in T cell activation and lineage differentiation. Semin. Immunol. 2016. 28: 514–524. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

