An Alanine Aminotransferase Is Required for Biofilm-Specific Resistance of Aspergillus fumigatus to Echinocandin Treatment
- PMID: 35254131
- PMCID: PMC9040767
- DOI: 10.1128/mbio.02933-21
An Alanine Aminotransferase Is Required for Biofilm-Specific Resistance of Aspergillus fumigatus to Echinocandin Treatment
Abstract
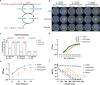
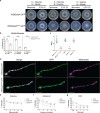
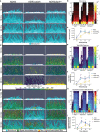
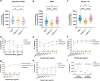
Alanine metabolism has been suggested as an adaptation strategy to oxygen limitation in organisms ranging from plants to mammals. Within the pulmonary infection microenvironment, Aspergillus fumigatus forms biofilms with steep oxygen gradients defined by regions of oxygen limitation. An alanine aminotransferase, AlaA, was observed to function in alanine catabolism and is required for several aspects of A. fumigatus biofilm physiology. Loss of alaA, or its catalytic activity, results in decreased adherence of biofilms through a defect in the maturation of the extracellular matrix polysaccharide galactosaminogalactan (GAG). Additionally, exposure of cell wall polysaccharides is also impacted by loss of alaA, and loss of AlaA catalytic activity confers increased biofilm susceptibility to echinocandin treatment, which is correlated with enhanced fungicidal activity. The increase in echinocandin susceptibility is specific to biofilms, and chemical inhibition of alaA by the alanine aminotransferase inhibitor β-chloro-l-alanine is sufficient to sensitize A. fumigatus biofilms to echinocandin treatment. Finally, loss of alaA increases susceptibility of A. fumigatus to in vivo echinocandin treatment in a murine model of invasive pulmonary aspergillosis. Our results provide insight into the interplay of metabolism, biofilm formation, and antifungal drug resistance in A. fumigatus and describe a mechanism of increasing susceptibility of A. fumigatus biofilms to the echinocandin class of antifungal drugs. IMPORTANCE Aspergillus fumigatus is a ubiquitous filamentous fungus that causes an array of diseases depending on the immune status of an individual, collectively termed aspergillosis. Antifungal therapy for invasive pulmonary aspergillosis (IPA) or chronic pulmonary aspergillosis (CPA) is limited and too often ineffective. This is in part due to A. fumigatus biofilm formation within the infection environment and the resulting emergent properties, particularly increased antifungal resistance. Thus, insights into biofilm formation and mechanisms driving increased antifungal drug resistance are critical for improving existing therapeutic strategies and development of novel antifungals. In this work, we describe an unexpected observation where alanine metabolism, via the alanine aminotransferase AlaA, is required for several aspects of A. fumigatus biofilm physiology, including resistance of A. fumigatus biofilms to the echinocandin class of antifungal drugs. Importantly, we observed that chemical inhibition of alanine aminotransferases is sufficient to increase echinocandin susceptibility and that loss of alaA increases susceptibility to echinocandin treatment in a murine model of IPA. AlaA is the first gene discovered in A. fumigatus that confers resistance to an antifungal drug specifically in a biofilm context.
Keywords: Aspergillus fumigatus; alanine; antifungal drugs; biofilm; biofilms; cell wall; echinocandin; echinocandins; extracellular matrix; galactosaminogalactan; hypoxia; metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sánchez ÓJ, Ospina DA, Montoya S. 2017. Compost supplementation with nutrients and microorganisms in composting process. Elsevier Ltd., New York, NY. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

