Multicenter Consensus Approach to Evaluation of Neonatal Hypotonia in the Genomic Era: A Review
- PMID: 35254387
- PMCID: PMC10134401
- DOI: 10.1001/jamaneurol.2022.0067
Multicenter Consensus Approach to Evaluation of Neonatal Hypotonia in the Genomic Era: A Review
Abstract
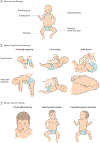
Importance: Infants with hypotonia can present with a variety of potentially severe clinical signs and symptoms and often require invasive testing and multiple procedures. The wide range of clinical presentations and potential etiologies leaves diagnosis and prognosis uncertain, underscoring the need for rapid elucidation of the underlying genetic cause of disease.
Observations: The clinical application of exome sequencing or genome sequencing has dramatically improved the timely yield of diagnostic testing for neonatal hypotonia, with diagnostic rates of greater than 50% in academic neonatal intensive care units (NICUs) across Australia, Canada, the UK, and the US, which compose the International Precision Child Health Partnership (IPCHiP). A total of 74% (17 of 23) of patients had a change in clinical care in response to genetic diagnosis, including 2 patients who received targeted therapy. This narrative review discusses the common causes of neonatal hypotonia, the relative benefits and limitations of available testing modalities used in NICUs, and hypotonia management recommendations.
Conclusions and relevance: This narrative review summarizes the causes of neonatal hypotonia and the benefits of prompt genetic diagnosis, including improved prognostication and identification of targeted treatments which can improve the short-term and long-term outcomes. Institutional resources can vary among different NICUs; as a result, consideration should be given to rule out a small number of relatively unique conditions for which rapid targeted genetic testing is available. Nevertheless, the consensus recommendation is to use rapid genome or exome sequencing as a first-line testing option for NICU patients with unexplained hypotonia. As part of the IPCHiP, this diagnostic experience will be collected in a central database with the goal of advancing knowledge of neonatal hypotonia and improving evidence-based practice.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

