Rituximab-treated patients with lymphoma develop strong CD8 T-cell responses following COVID-19 vaccination
- PMID: 35254660
- PMCID: PMC9111866
- DOI: 10.1111/bjh.18149
Rituximab-treated patients with lymphoma develop strong CD8 T-cell responses following COVID-19 vaccination
Abstract
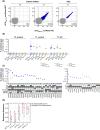
B-cell depletion induced by anti-cluster of differentiation 20 (CD20) monoclonal antibody (mAb) therapy of patients with lymphoma is expected to impair humoral responses to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) vaccination, but effects on CD8 T-cell responses are unknown. Here, we investigated humoral and CD8 T-cell responses following two vaccinations in patients with lymphoma undergoing anti-CD20-mAb therapy as single agent or in combination with chemotherapy or other anti-neoplastic agents during the last 9 months prior to inclusion, and in healthy age-matched blood donors. Antibody measurements showed that seven of 110 patients had antibodies to the receptor-binding domain of the SARS-CoV-2 Spike protein 3-6 weeks after the second dose of vaccination. Peripheral blood CD8 T-cell responses against prevalent human leucocyte antigen (HLA) class I SARS-CoV-2 epitopes were determined by peptide-HLA multimer analysis. Strong CD8 T-cell responses were observed in samples from 20/29 patients (69%) and 12/16 (75%) controls, with similar median response magnitudes in the groups and some of the strongest responses observed in patients. We conclude that despite the absence of humoral immune responses in fully SARS-CoV-2-vaccinated, anti-CD20-treated patients with lymphoma, their CD8 T-cell responses reach similar frequencies and magnitudes as for controls. Patients with lymphoma on B-cell depleting therapies are thus likely to benefit from current coronavirus disease 2019 (COVID-19) vaccines, and development of vaccines aimed at eliciting T-cell responses to non-Spike epitopes might provide improved protection.
Keywords: CD8 T-cell response; anti-CD20 antibody; coronavirus disease 2019 (COVID-19) vaccination; humoral response; lymphoma; severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) epitopes.
© 2022 The Authors. British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
A patent application was filed by the institutional technology transfer office Inven2 covering SARS‐CoV‐2 epitopes (Inventors: Johanna Olweus, Fridtjof Lund‐Johansen, Saskia Meyer, Isaac Blaas, Even Holth Rustad). All other co‐authors confirm no competing interest.
Figures

References
-
- Desai A, Gupta R, Advani S, Ouellette L, Kuderer NM, Lyman GH, et al. Mortality in hospitalized patients with cancer and coronavirus disease 2019: a systematic review and meta‐analysis of cohort studies. Cancer. 2021;127:1459–68. - PubMed
-
- McLaughlin P, Grillo‐Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti‐CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four‐dose treatment program. J Clin Oncol. 1998;16:2825–33. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

