Probing the Mechanism of Isonitrile Formation by a Non-Heme Iron(II)-Dependent Oxidase/Decarboxylase
- PMID: 35254829
- PMCID: PMC8986608
- DOI: 10.1021/jacs.1c12891
Probing the Mechanism of Isonitrile Formation by a Non-Heme Iron(II)-Dependent Oxidase/Decarboxylase
Abstract
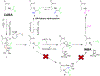
The isonitrile moiety is an electron-rich functionality that decorates various bioactive natural products isolated from diverse kingdoms of life. Isonitrile biosynthesis was restricted for over a decade to isonitrile synthases, a family of enzymes catalyzing a condensation reaction between l-Trp/l-Tyr and ribulose-5-phosphate. The discovery of ScoE, a non-heme iron(II) and α-ketoglutarate-dependent dioxygenase, demonstrated an alternative pathway employed by nature for isonitrile installation. Biochemical, crystallographic, and computational investigations of ScoE have previously been reported, yet the isonitrile formation mechanism remains obscure. In the present work, we employed in vitro biochemistry, chemical synthesis, spectroscopy techniques, and computational simulations that enabled us to propose a plausible molecular mechanism for isonitrile formation. Our findings demonstrate that the ScoE reaction initiates with C5 hydroxylation of (R)-3-((carboxymethyl)amino)butanoic acid to generate 1, which undergoes dehydration, presumably mediated by Tyr96 to synthesize 2 in a trans configuration. (R)-3-isocyanobutanoic acid is finally generated through radical-based decarboxylation of 2, instead of the common hydroxylation pathway employed by this enzyme superfamily.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


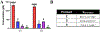


References
-
- Wang L; Zhu M; Zhang Q; Zhang X; Yang P; Liu Z; Deng Y; Zhu Y; Huang X; Han L; Li S; He J Diisonitrile Natural Product SF2768 Functions As a Chalkophore That Mediates Copper Acquisition in Streptomyces Thioluteus. ACS Chem. Biol 2017, 12 (12), 3067–3075. - PubMed
-
- Gloer JB; Rinderknecht BL Nominine : A New Insecticidal Indole Diterpene from the Sclerotia of Aspergillus Nomius. J. Org. Chem 1989, No. 6, 2530–2532.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

