Phase I Study of Safety, Tolerability, and Efficacy of Tebentafusp Using a Step-Up Dosing Regimen and Expansion in Patients With Metastatic Uveal Melanoma
- PMID: 35254876
- PMCID: PMC9177239
- DOI: 10.1200/JCO.21.01805
Phase I Study of Safety, Tolerability, and Efficacy of Tebentafusp Using a Step-Up Dosing Regimen and Expansion in Patients With Metastatic Uveal Melanoma
Abstract
Purpose: This phase I study aimed to define the recommended phase II dose (RP2D) of tebentafusp, a first-in-class T-cell receptor/anti-CD3 bispecific protein, using a three-week step-up dosing regimen, and to assess its safety, pharmacokinetics, pharmacodynamics, and preliminary clinical activity in patients with metastatic uveal melanoma (mUM).
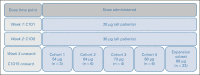
Methods: In this open-label, international, phase I/II study, HLA-A*02 or HLA-A*02:01+ patients with mUM received tebentafusp 20 μg once in week 1 and 30 μg once in week 2. Dose escalation (starting at 54 μg) began at week 3 in a standard 3 + 3 design to define RP2D. Expansion-phase patients were treated at the RP2D (20-30-68 μg). Blood and tumor samples were collected for pharmacokinetics/pharmacodynamics assessment, and treatment efficacy was evaluated for all patients with baseline efficacy data as of December 2017.
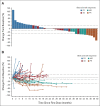
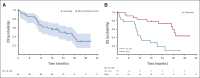
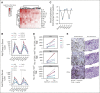
Results: Between March 2016 and December 2017, 42 eligible patients who failed a median of two previous treatments were enrolled: 19 in the dose escalation cohort and 23 in an initial dose expansion cohort. Of the dose levels investigated, 68 μg was identified as the RP2D. Most frequent treatment-emergent adverse events regardless of attribution were pyrexia (91%), rash (83%), pruritus (83%), nausea (74%), fatigue (71%), and chills (69%). Toxicity attenuated following the first three doses. The overall response rate was 11.9% (95% CI, 4.0 to 25.6). With a median follow-up of 32.4 months, median overall survival was 25.5 months (range, 0.89-31.1 months) and 1-year overall survival rate was 67%. Treatment was associated with increased tumor T-cell infiltration and transient increases in serum inflammatory mediators.
Conclusion: Using a step-up dosing regimen of tebentafusp allowed a 36% increase in the RP2D compared with weekly fixed dosing, with a manageable side-effect profile and a signal of efficacy in mUM.
Conflict of interest statement
Figures

References
-
- Milam RW, Daniels AB: Uveal melanoma, in Riker AI. (ed): Melanoma: A Modern Multidisciplinary Approach. Cham, Switzerland, Springer International Publishing, 2018, pp 273-312
-
- Stang A, Parkin DM, Ferlay J, et al. : International uveal melanoma incidence trends in view of a decreasing proportion of morphological verification. Int J Cancer 114:114-123, 2005 - PubMed
-
- Virgili G, Gatta G, Ciccolallo L, et al. : Incidence of uveal melanoma in Europe. Ophthalmology 114:2309-2315, 2007 - PubMed
-
- Kujala E, Makitie T, Kivela T: Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci 44:4651-4659, 2003 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

