Phosphonate production by marine microbes: Exploring new sources and potential function
- PMID: 35254902
- PMCID: PMC8931226
- DOI: 10.1073/pnas.2113386119
Phosphonate production by marine microbes: Exploring new sources and potential function
Abstract
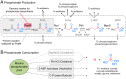
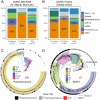
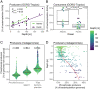
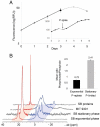
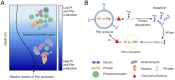
SignificancePhosphonates are a class of phosphorus metabolites characterized by a highly stable C-P bond. Phosphonates accumulate to high concentrations in seawater, fuel a large fraction of marine methane production, and serve as a source of phosphorus to microbes inhabiting nutrient-limited regions of the oligotrophic ocean. Here, we show that 15% of all bacterioplankton in the surface ocean have genes phosphonate synthesis and that most belong to the abundant groups Prochlorococcus and SAR11. Genomic and chemical evidence suggests that phosphonates are incorporated into cell-surface phosphonoglycoproteins that may act to mitigate cell mortality by grazing and viral lysis. These results underscore the large global biogeochemical impact of relatively rare but highly expressed traits in numerically abundant groups of marine bacteria.
Keywords: Prochlorococcus; biogeochemistry; marine; phosphonate; phosphorus.
Conflict of interest statement
The authors declare no competing interest.
Figures
References
-
- Dyhrman S. T., “Nutrients and their acquisition: Phosphorus physiology in microalgae” in The Physiology of Microalgae, Borowitzka M. A., Beardall J., Raven J. A., Eds. (Springer International Publishing, 2016), pp. 155–183.
-
- Van Mooy B. A. S., Devol A. H., Assessing nutrient limitation of Prochlorococcus in the North Pacific subtropical gyre by using an RNA capture method. Limnol. Oceanogr. 53, 78–88 (2008).
-
- Van Mooy B. A. S., et al. , Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature 458, 69–72 (2009). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

