Clinical and In Vitro Evidence Favoring Immunoglobulin Treatment of a Chronic Norovirus Infection in a Patient With Common Variable Immunodeficiency
- PMID: 35255136
- PMCID: PMC9650502
- DOI: 10.1093/infdis/jiac085
Clinical and In Vitro Evidence Favoring Immunoglobulin Treatment of a Chronic Norovirus Infection in a Patient With Common Variable Immunodeficiency
Abstract
Background: Immunocompromised individuals can become chronically infected with norovirus, but effective antiviral therapies are not yet available.
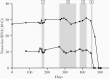
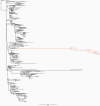
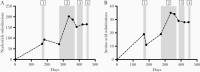
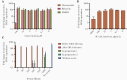
Methods: Treatments with nitazoxanide, ribavirin, interferon alpha-2a, and nasoduodenally administered immunoglobulins were evaluated sequentially in an immunocompromised patient chronically infected with norovirus. In support, these components were also applied to measure norovirus inhibition in intestinal enteroid cultures in vitro. Viral RNA levels were determined in fecal and plasma samples during each treatment and viral genomes were sequenced.
Results: None of the antivirals resulted in a reduction of viral RNA levels in feces or plasma. However, during ribavirin treatment, there was an increased accumulation of virus genome mutations. In vitro, an effect of interferon alpha-2a on virus replication was observed and a genetically related strain was neutralized effectively in vitro using immunoglobulins and post-norovirus-infection antiserum. In agreement, after administration of immunoglobulins, the patient cleared the infection.
Conclusions: Intestinal enteroid cultures provide a relevant system to evaluate antivirals and the neutralizing potential of immunoglobulins. We successfully treated a chronically infected patient with immunoglobulins, despite varying results reported by others. This case study provides in-depth, multifaceted exploration of norovirus treatment that can be used as a guidance for further research towards norovirus treatments.
Keywords: VirCapSeq; enteroids; human intestinal organoids; immunocompromised; immunoglobulins; interferon alpha-2a; next-generation sequencing; nitazoxanide; norovirus; ribavirin.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Siebenga JJ, Beersma MF, Vennema H, van Biezen P, Hartwig NJ, Koopmans M.. High prevalence of prolonged norovirus shedding and illness among hospitalized patients: a model for in vivo molecular evolution. J Infect Dis 2008; 198:994–1001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

