Effect of Dupilumab in Korean Patients With Uncontrolled Moderate-to-Severe Asthma: A LIBERTY ASTHMA QUEST Sub-analysis
- PMID: 35255536
- PMCID: PMC8914602
- DOI: 10.4168/aair.2022.14.2.182
Effect of Dupilumab in Korean Patients With Uncontrolled Moderate-to-Severe Asthma: A LIBERTY ASTHMA QUEST Sub-analysis
Abstract
Purpose: To assess the effect of dupilumab on the annualized severe exacerbation rates, change in forced expiratory volume at first second (FEV1), overall asthma control and health-related quality of life in Korean patients from the LIBERTY ASTHMA QUEST study.
Methods: Of the 1,902 patients enrolled in the LIBERTY ASTHMA QUEST study, a phase-3, randomized, double-blind, placebo-controlled, parallel-group study on dupilumab, 74 (4%) were Korean. The patients were randomly assigned to 4 treatment groups (2:2:1:1). The sub-analysis reported herewith was performed with the pooled groups of dupilumab and placebo from the 4 original treatment groups in the LIBERTY ASTHMA QUEST study. The efficacy endpoints were annualized rate of severe exacerbation events during the 52-week study period and changes from baseline in pre-bronchodilator FEV1 in week 12. Asthma control, asthma quality of life and the effect of treatment on the levels of type 2 inflammatory biomarkers were assessed. The safety profile was also evaluated.
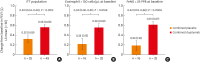
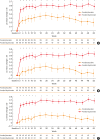
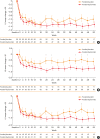
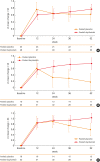
Results: In Korean patients, annualized severe exacerbation rates were reduced with dupilumab (n = 49) compared to placebo (n = 25) (0.259 vs 1.942) during the 52-week treatment period. The relative risk reduction with dupilumab was 87% (P < 0.001). Improvements in pre-bronchodilator FEV1 (mean difference of 0.24 L, P = 0.021) were observed in week 12 in dupilumab-treated patients. Additionally, improvements in asthma control and asthma-related quality of life were observed; the FeNO and serum immunoglobulin E levels were reduced. The incidence of adverse events and serious adverse events was comparable between the dupilumab and placebo group. A total of 11 patients from the dupilumab group reported 63 injection site reactions.
Conclusions: Dupilumab, as an add-on therapy in severe asthma, is efficacious and has an acceptable safety profile in Korean patients.
Trial registration: ClinicalTrials.gov Identifier: NCT02414854.
Keywords: Exacerbation; asthma; biological products; forced expiratory volume; quality of life; randomized controlled trial.
Copyright © 2022 The Korean Academy of Asthma, Allergy and Clinical Immunology • The Korean Academy of Pediatric Allergy and Respiratory Disease.
Conflict of interest statement
Chin Kook Rhee, Heung-Woo Park, You Sook Cho are on advisory panel on Sanofi Aventis Korea Jung-Won Park has no conflict of interest to declare.
Figures

Similar articles
-
Liberty Asthma QUEST: Phase 3 Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate Dupilumab Efficacy/Safety in Patients with Uncontrolled, Moderate-to-Severe Asthma.Adv Ther. 2018 May;35(5):737-748. doi: 10.1007/s12325-018-0702-4. Epub 2018 May 3. Adv Ther. 2018. PMID: 29725983 Free PMC article. Clinical Trial.
-
Dupilumab efficacy and safety in Japanese patients with uncontrolled, moderate-to-severe asthma in the phase 3 LIBERTY ASTHMA QUEST study.Allergol Int. 2020 Oct;69(4):578-587. doi: 10.1016/j.alit.2020.04.002. Epub 2020 May 20. Allergol Int. 2020. PMID: 32444306 Clinical Trial.
-
Dupilumab efficacy and safety in Latin American patients with uncontrolled, moderate-to-severe asthma: phase 3 LIBERTY ASTHMA QUEST study.J Asthma. 2023 May;60(5):981-990. doi: 10.1080/02770903.2022.2115927. Epub 2022 Oct 17. J Asthma. 2023. PMID: 36066123 Clinical Trial.
-
Dupilumab safety and efficacy in uncontrolled asthma: a systematic review and meta-analysis of randomized clinical trials.J Asthma. 2019 Oct;56(10):1110-1119. doi: 10.1080/02770903.2018.1520865. Epub 2018 Oct 1. J Asthma. 2019. PMID: 30273510
-
Dupilumab in the Treatment of Moderate to Severe Asthma: An Evidence-Based Review.Curr Ther Res Clin Exp. 2019 Oct 28;91:45-51. doi: 10.1016/j.curtheres.2019.100571. eCollection 2019. Curr Ther Res Clin Exp. 2019. PMID: 31871508 Free PMC article. Review.
Cited by
-
Long-term Safety and Efficacy of Dupilumab in Patients With Uncontrolled, Moderate-to-Severe Asthma Recruited From Korean Centers: A Subgroup Analysis of the Phase 3 LIBERTY ASTHMA TRAVERSE Trial.Allergy Asthma Immunol Res. 2024 Jul;16(4):372-386. doi: 10.4168/aair.2024.16.4.372. Allergy Asthma Immunol Res. 2024. PMID: 39155737 Free PMC article.
-
Dupilumab Treatment for Asthma: On the Road to a New Horizon Beyond Ethnic Differences?Allergy Asthma Immunol Res. 2022 Mar;14(2):147-150. doi: 10.4168/aair.2022.14.2.147. Allergy Asthma Immunol Res. 2022. PMID: 35255532 Free PMC article. No abstract available.
-
Long-term efficacy of anti-IL-4 receptor antibody in a patient with aspirin-exacerbated respiratory disease and IgG4-related disease.Allergy Asthma Clin Immunol. 2023 Aug 5;19(1):67. doi: 10.1186/s13223-023-00825-z. Allergy Asthma Clin Immunol. 2023. PMID: 37543606 Free PMC article.
-
Cost-effectiveness analysis of dupilumab among patients with uncontrolled severe asthma using LIBERTY ASTHMA QUEST Korean data.Health Econ Rev. 2024 Aug 26;14(1):67. doi: 10.1186/s13561-024-00532-4. Health Econ Rev. 2024. PMID: 39186143 Free PMC article.
-
Real-World Effectiveness of Biologics in Patients With Severe Asthma: Analysis of the KoSAR.Allergy Asthma Immunol Res. 2024 May;16(3):253-266. doi: 10.4168/aair.2024.16.3.253. Allergy Asthma Immunol Res. 2024. PMID: 38910283 Free PMC article.
References
-
- U.S. Food & Drug Administration. Dupixent prescribing information [Internet] Silver Spring (MD): U.S. Food & Drug Administration; 2017. [cited 2020 Nov 3]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761055s014lbl.pdf.
-
- European Medicines Agency. Prescribing information. Dupixent [Internet] Amsterdam: European Medicines Agency; 2017. [cited 2021 Sep 14]. Available from: https://www.ema.europa.eu/en/documents/product-information/dupixent-epar....

